Keywords
Conventional tablet, Sustained release, Controlled release, Polymer, Matrix tablet.
Introduction
Oral Drug Delivery Systems
The term “Drug Delivery” covers a very extensive range of techniques used to deliver therapeutic agents into the human body. Drugs are administered with a main aim of curing patient ailments. Drugs are never administered in their pure form but are converted in a suitable formulation so that its onset and intensity of action as well as total duration of action can be checked. Among the various routes of drug delivery oral route is most widely used route of drug delivery. But conventional dosage form offers few limitations which could be resolved by modifying the existing dosage form [1,2].
Limitations of conventional oral dosage form:
• Poor patient compliance; chances of dose missing.
• See-saw fluctuations.
• Multiple drug therapy enhances the risk of toxicity as well as overall cost of treatment [3].
Approaches to overcome these limitations:
• Development of new, better and safer drugs with long half-life and large therapeutic indices.
• Effective and safer use of existing drugs through concepts and techniques of controlled and targeted drug delivery systems [4,5].
The first approach has many disadvantages, however second approach can be used widely.
An ideal controlled drug delivery system is that which delivers the drug at a specific rate locally or systemically for a specified period of time with minimum fluctuation in plasma drug concentration, reduced toxicity and maximum efficiency [6].
• Anatomy and physiology of GIT.
• Pharmacodynamics and pharmacokinetic profile of drug.
• Physicochemical characteristics of drug and delivery mode under study [3,7].
The oral controlled release formulations have been developed for those therapeutic agents that are easily absorbed from the G.I.T, having a shorter half life, eliminated quickly from the blood circulation, narrow absorption window as these will release the drug slowly into the G.I.T [4,8].
Controlled release dosage forms cover a wide range of prolonged action formulations which provide continuous release of drug at the specific site for a predetermined time at a predetermined rate [9].
There are several terms used interchangeably viz. controlled release, programmed release, sustained release, prolonged release, timed release, extended release etc. The most important objective for the development of these systems is to furnish an extended duration of action and thus assure greater patient compliance [5-12].
Sustained Release Dosage Form
Sustained release dosage form is defined as well characterized and reproducible dosage form, which is designed to control drug release profile at a specified rate to achieve desired drug concentration either in blood plasma or at target site [13].
This system will provide actual therapeutic control that would be temporal (time related), spatial (site related) or both [6,14].
Advantages of sustained drug delivery system:
• Reduced see-saw fluctuations.
• Total amount of dose decreases.
• Improved patient compliance.
• Increased safety of drugs [15-19].
Disadvantages of sustained drug delivery system:
• Chances of dose dumping.
• Dose retrieval is difficult.
• High cost of formulation.
• Need for additional patient education.
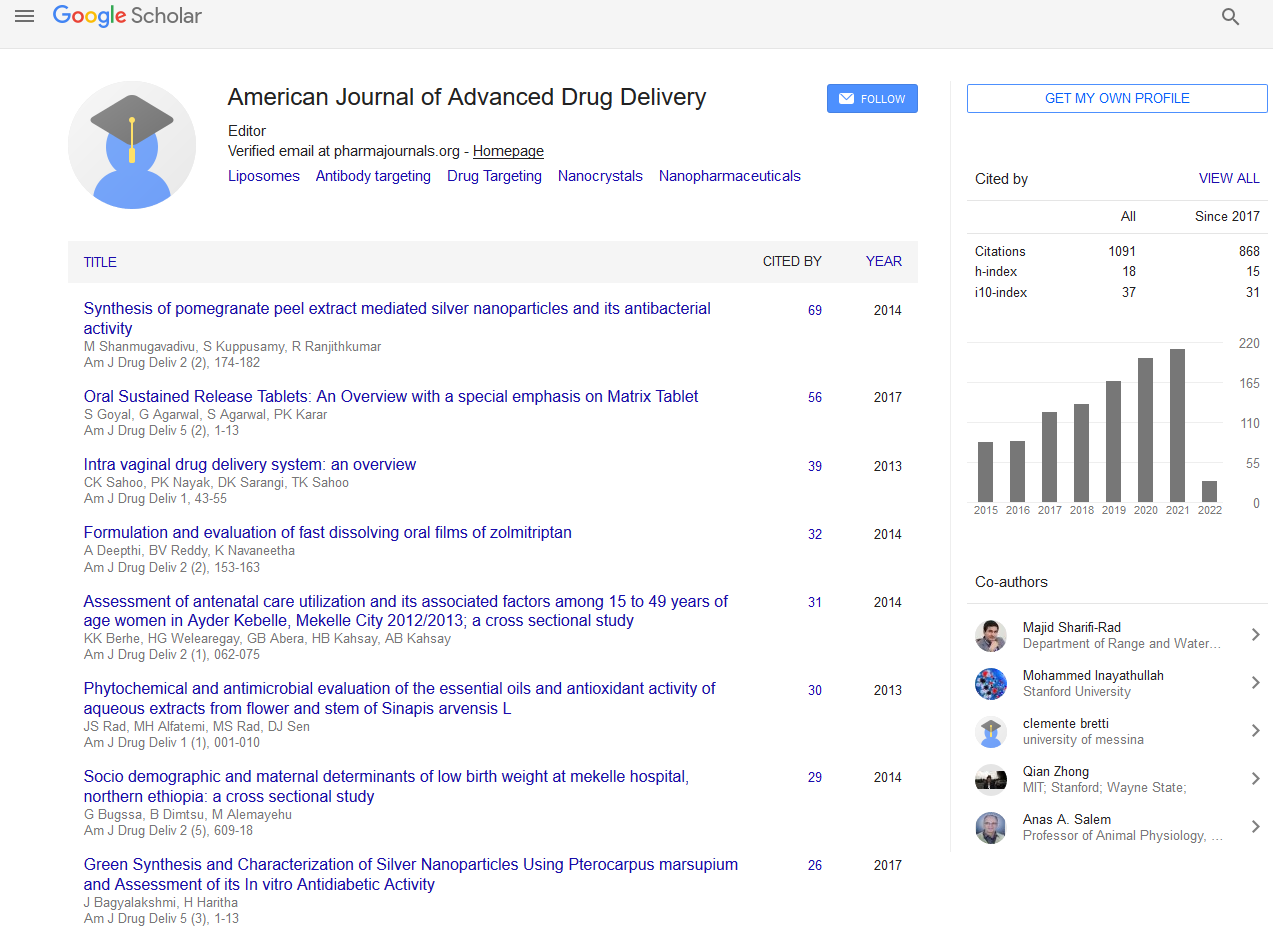
• Reduced potential for accurate dose adjustment [20,21] (Figure 1).
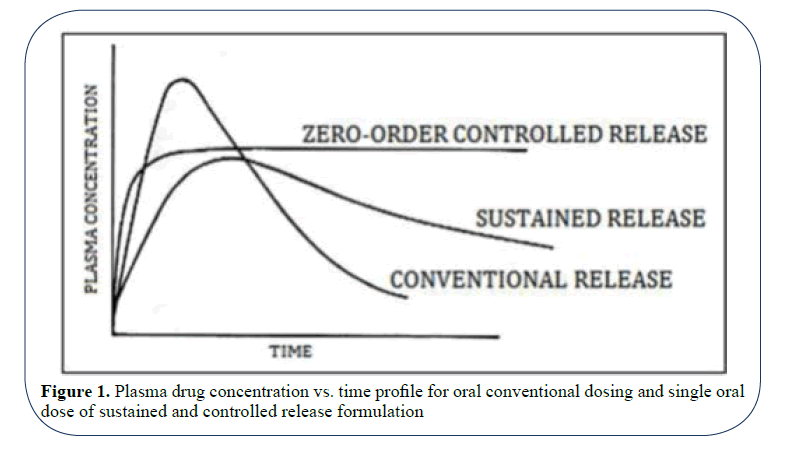
Figure 1: Plasma drug concentration vs. time profile for oral conventional dosing and single oral dose of sustained and controlled release formulation
The performance of a drug presented as a controlled/sustained release system depends upon its:
• Release from the formulation.
• Movement within the body during its passage to the site of action [22].
The desired biopharmaceutical properties of a drug to be used in sustained drug delivery system are discussed below:
Molecular weight: Lower the molecular weight faster and more complete will be the absorption. For the drugs absorbed by pore transport mechanism, the molecular size threshold is 150 Daltons for spherical compounds and 400 Daltons for linear compounds. The upper limit for the molecular size is 600 Daltons for passive diffusion.
Aqueous solubility of the drug: Drugs with good aqueous solubility are good candidate for sustained release dosage form rather than the candidates having poor aqueous solubility.
Apparent partition coefficient of drug: Greater the apparent partition coefficient of the drug, greater is the rate and extent of absorption.
Drug pka and ionization at physiological pH: The pka range for acidic drugs is 3.0-7.5 and that for basic drugs is 7.0-11.0. Aqueous solubility of weak acids and bases is governed by pka of compound and pH of the medium. According to pH theory, the absorption of acidic drugs is favoured in acidic environment and that of basic drugs in basic environment. Hence, the release of the ionizable drugs must be programmed in accordance with pH variations across the GIT. So, the drugs that exist largely in ionized form serves as poor candidates for sustained release dosage form [23].
Drug stability: Drugs unstable in GI environment are poor candidates for oral sustained release system.
Biopharmaceutical aspects of route of administration: Oral and parental (i.m.) routes followed by transdermal are most popular. Routes of minor importance in sustained drug delivery are buccal/sublingual, nasal, rectal, ocular and pulmonary [7,24].
A detailed knowledge of ADME characteristics of drug is essential in the design of sustained release product. An optimum range of given pharmacokinetic parameter of a drug is necessary beyond which controlled/sustained delivery is difficult [25-30] (Tables 1 and 2).
Table 1. Physicochemical parameters for drug selection
| Parameters |
Criteria |
| Molecular size |
Less than 600 Daltons |
| Aqueous solubility |
More than 0.1mg/ml |
| Partition coefficient Ko/w |
1-2 |
| Dissociation constant pka |
Acidic drugs, pka>2.5
Basic drugs, pka<11.0 |
| Absorption mechanism |
Passive |
| Stability in GI milieu |
Stable at both gastric and intestinal pH |
| Ionization at physiological pH |
Not more than 95% |
Table 2. Pharmacokinetic parameters for drug selection
| Parameters |
Comment |
| Elimination half life |
Between 2-6 hrs |
| Absolute bioavailability |
> 75% or more |
| Absorption rate constant (Ka) |
High |
| Metabolism Rate |
Not too High |
| Total clearance |
Should not depend on dose |
| Therapeutic concentration (Css) |
Lower Css and small Vd |
Formulation Strategies for Oral Sustained Release System
Diffusion Sustained Release
Dissolution Sustained Release
pH Dependent System
Altered Density System
Osmotic Pump System
Ion Exchange System [31]
Types of diffusion sustained system:
• Swellable matrix.
• Reservoir/Laminate matrix.
Types of dissolution sustained system:
• Matrix/Monolith Dissolution System.
• Encapsulation/Coating/Reservoir System.
Types of altered density system:
• High Density System.
• Low Density System.
• Muco Adhesive System8,32.
Diffusion sustained system: These systems are those where the rate controlling step is not the dissolution rate of the drug but diffusion of the dissolved drug molecule. Depending upon the mechanism such system can be classified as:
Porous membrane controlled system: In these type of system the rate controlling element is a water insoluble non swellable polymer like ethyl cellulose, polymethaacrylate etc. which controls the drug release through the micro pores present in their membrane or matrix structure [33-35] (Figure 2).
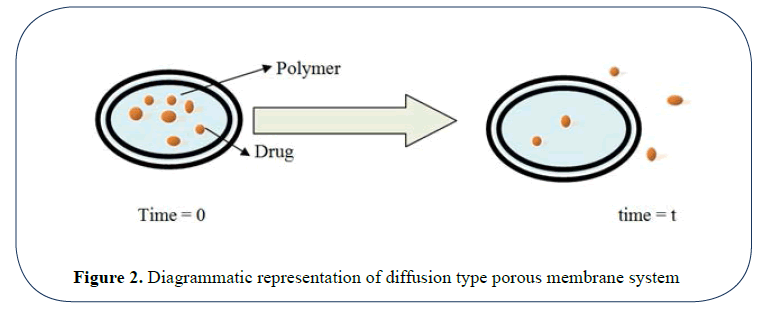
Figure 2: Diagrammatic representation of diffusion type porous membrane system
Advantages
• Can provide zero order drug release.
Disadvantages
• High cost per dosage unit.
• In case of dose dumping toxicity can take place.
Porous matrix controlled system: In these type of system the rate controlling element is a water swellable material ( hydrophilic polymers and gums) like alginates, xanthan gum, locust bean gum, HPMC etc. or a non swellable water insoluble polymer like ethyl cellulose [36] (Figure 3).
Figure 3: Diagrammatic representation of diffusion type porous membrane system
Advantages
• Cost effective.
• Easy to fabricate.
• Drug could be protected from hydrolysis or other changes in GIT, so enhanced stability.
• Compounds with high molecular weight could be formulated.
Disadvantages
• Release rate is affected by presence of food.
• Matrix must be removed after the release of drug.
Dissolution sustained system
The drugs with slow solubility are suitable candidates for this system and for the drugs having high solubility the dissolution is decreased by conversion into a suitable salt or derivative [37,38].
Drug present in this system may be of two types:
• Drugs with inherently slow dissolution rate. e.g. griseofulvin, digoxin, nifedipine etc.
• Drugs that transforms into a slow dissolving form on coming in contact with GI fluids. e.g. Ferrous sulphate.
• Drugs having high aqueous solubility and dissolution rate [9,39].
These systems can further be categorized as:
Coating dissolution system: In this type of system the drug particles are coated with polymers like cellulose, polymethacrylates, PEGs etc. The resulting pellets are compressed as tablets. The dissolution rate of the coat depends upon thickness and solubility of coat [40] (Figure 4).
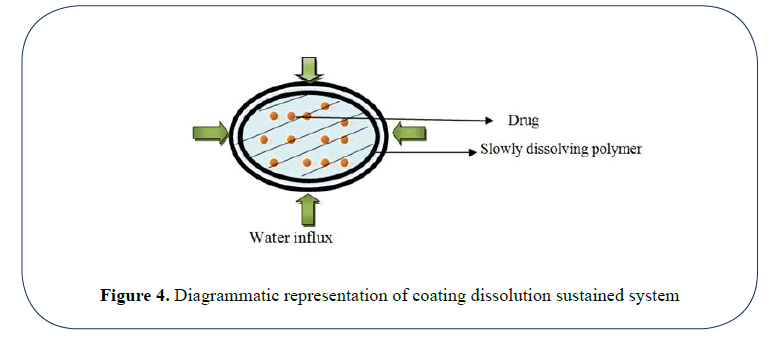
Figure 4: Diagrammatic representation of coating dissolution sustained system
Soluble matrix system: These systems are also known as monoliths as the drug is homogenously dispersed in a rate controlling medium. Waxes like bee wax, carnauba wax etc. are used for controlling the dissolution rate.
The rate of dissolution is controlled by either of following mechanisms:
• Altering the rate of fluid penetration into tablet by altering the porosity of tablet.
• Decreasing the wettability of tablet.
• Slow dissolution rate of polymer [6,7] (Figure 5).
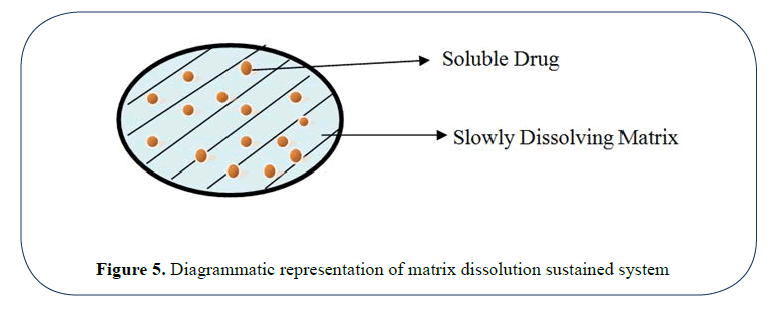
Figure 5: Diagrammatic representation of matrix dissolution sustained system
Ion exchange resins: Based upon the principle that GIT has a relatively constant level of ions, this type of system has developed for controlling the rate of delivery of ionisable or ionic drugs. Such a system can be prepared by incubating the drug resin solution or by passing the drug solution through a column containing exchange resin. A cationic drug is complexed with a resin containing SO3- group and for anionic drug resin containing N(CH3)3 group is used. In the GIT hydronium and chloride ions diffuses into the sustained release tablet and interact with drugresin compex to trigger the release of drug.
+Types of ion exchange resins:
Cationic exchange resin: Contains acidic functional group.
Anion exchange resin: Contains basic functional group.
These systems prevent dose dumping as they have better drug retaining properties. So, chances of toxicity are reduced. Moreover, the polymeric and ionic property of ion exchange resin makes the drug release more uniform than that of simple matrices [41].
Method using osmotic pump: Osmotic systems are based on the principle of osmosis. Such systems release the drugs at a constant zero order rate. These systems are popularly known as OROS.
The system consist of a drug core and an osmotically active substance (osmogen) like mannitol surrounded by a semipermeable membrane coating with an orifice of 0.4 mm made by laser beam to facilitate drug exit. When exposed to GI Fluids, water flows though semipermeable membrane, under the influence of osmotic force of osmogen the drug release is facilitated via orifice.
These systems could be of two types:
In type-A system drug splution along with osmogen is surrounded by a semi permeable membrane.
In type-B system, the drug solution is present in a semi permeable membrane surrounded by the electrolytes (Figure 6).
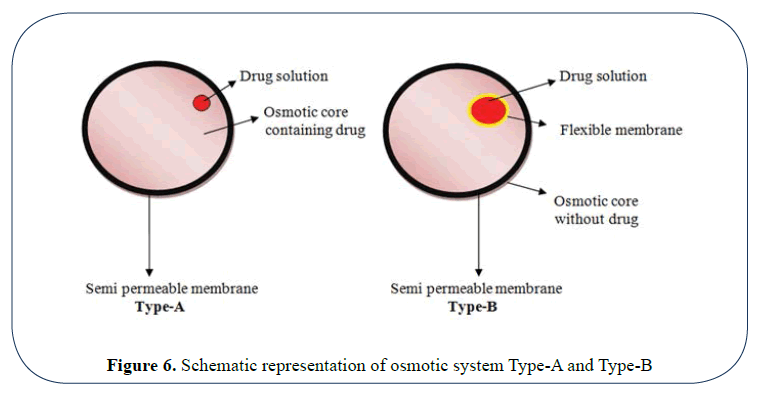
Figure 6: Schematic representation of osmotic system Type-A and Type-B
pH independent formulations: These systems are designed for acid-labile drugs or drugs irritating GIT mucosa and targeting their delivery to the intestinal tract. It is fabricated by coating the core of tablet with a combination of intestinal fluid insoluble polymer (ethyl cellulose) and intestinal fluid soluble polymer (HPMCP). The coating membrane resists the dissolution of drug in stomach at acidic pH. After gastric emptying the system travels to small intestine. At a pH above 5 the intestine soluble component dissolves; thereby producing a porous membrane that controls the release of drug from the core of the tablet.
Altered density formulations: The transit time of GI contents is usually less than 24 hours. This is major limiting factor in design of sustained release formulation. If the residence time of drug in stomach or intestine is prolonged the frequency of dosing can further be reduced. This could be achieved by altering the density of drug particles, using mucoadhesive polymers or by altering the size of dosage form.
Altered density formulation can further be classified as:
i) High density approach
The density of GI fluids is about 1.4 g/ cc so the drug particles having density greater than this value usually 1.6 g/cc can be used for this purpose. These systems have prolonged residence time and not affected by presence of food. Iron oxide and barium sulfate can be used for this purpose.
ii) Low density approach
These pellets have density lower than that of GI fluids. So, such tablets tend to float on gastric juice for an extended time period thereby slowing down the drug release. Such system can be formulated by granulating a drug with 20- 80% of hydrogel like HPMC, HPC and HEC. On contact with GI fluids, tablets swells and form a diffusible gel barrier that lowers the density of the system lower than 1; thereby allowing it to float.
Matrix Tablet
Matrix tablet is defined as “Oral solid dosage form in which active pharmaceutical ingredient is uniformly dispersed throughout polymeric matrices (hydrophilic or hydrophobic) which retards the drug release rate.
This approach is widely used for formulating the sustained release tablets. The mechanism involved in the drug release is either dissolution controlled or diffusion controlled.
Advantages of matrix system:
• Easy to manufacture.
• Cost effective.
• Improved patient compliance.
• Sustained release formulations avoid the high blood concentration.
• Reduce drug toxicity by slowing down drug absorption.
• Enhanced drug stability in GI milieu.
• Minimize the local and systemic side effects.
• No see-saw fluctuations in plasma drug concentration profile.
• Less amount of drug is required.
• Temporal effects can be provided. e.g. morning relief of arthritis through bed time dosing.
Disadvantages of matrix tablets:
• Matrix needs to be removed after drug release.
• Costly in comparison to conventional dosage form.
• Presence of food and gut transition time can affect the release rate [10].
Classification of Matrix Tablets
A) On the basis of retardant material used matrix can be divided into 5 types:
Hydrophobic matrices (plastic matrices):
In this technique hydrophobic inert polymer are used as release retarding matrix material. The drug is mixed with the hydrophobic inert polymer (e.g. polyethylene, poly vinyl chloride, ethyl cellulose) and then compressed into tablet. The drug is entrapped between the network channels of polymer particles thereby sustaining the release of drug.
Lipid matrices:
Lipid material is used as release retardant (e.g. carnauba wax in combination with stearyl alcohol). Mechanism involved in drug release includes both pore diffusion and matrix erosion.
Hydrophilic matrices:
In this type of system a variety of hydrophilic polymers can be used, such systems are also known as swellable matrices. These polymers are more preferred than former ones as they are cost effective and a desirable drug profile can be easily obtained.
Classification of hydrophilic polymer matrices:
• Cellulose derivatives: Methyl cellulose 400 and 4000cPs; Hydroxy ethyl cellulose, Hydroxy propyl methyl cellulose (HPMC) 25, 100, 4000 and 15000cPs and Sodium carboxy methyl cellulose.
• Non cellulose natural and semi-synthetic polymers: Agar-Agar; alginates; carob gum; molasses; polysaccharides of galactose and mannose; chitosan and modified starches.
• Polymer of acrylic acid: carbopol-934.
Biodegradable polymers:
These consist of biodegradable polymers that are degraded either by enzymatic or non enzymatic process into by products which are excreted out from the body e.g. Polyanhydrides, proteins, polysaccharides.
Mineral matrices: Species of sea weeds like alginic acid are used as release retardants [7,11] (Tables 3 and 4).
Table 3. Classification of matrix tablets on the basis of porosity of matrix
| S No. |
Type |
Pore size |
Mechanism involved |
| 1 |
Macro Porous System |
0.1-1µm |
Diffusion through pore of matrix |
| 2 |
Micro Porous System |
50-200Å |
Diffusion through pore of matrix |
| 3 |
Non Porous System |
---------------- |
Diffusion through network meshes |
Table 4. Polymers used in matrix tablet
| S No. |
Polymer Class |
Example |
| 1 |
Hydrogels |
Cross-linked polyvinyl alcohol (PVA), Cross-linked polyvinyl pyrrolidone(PVP), Polyethylene oxide (PEO) |
| 2 |
Soluble polymers |
Polyvinylpyrrolidone (PVP), Hydroxypropyl methyl cellulose (HPMC) |
| 3 |
Biodegradable polymers |
Polylactic acid (PLA), Polyglycolic acid (PGA) |
| 4 |
Mucoadhesive polymers |
Polyacrylic acid, Tragacanth, Methyl cellulose, Pectin |
| 5 |
Non-Biodegradable Polymers |
Cellulose acetate (CA), Ethyl cellulose (EC) |
| 6 |
Natural gums |
Guar gum, Karaya gum, Locust bean gum |
Mechanism of drug release: The mechanism involved in drug release includes either diffusion or dissolution. On exposure with aqueous solution hydration of matrix takes place as a result it swells to block up existing pores, dissolution of the contents takes place. Due to gel formation a viscous solution is formed which give rise to a positive pressure which opposes the liquid entry and causes the disintegration of matrix.
The swelling of the matrix and consequent drug release by diffusion from the matrix and erosion of the matrix is as shown in (Figures 7 and 8) [11,12].
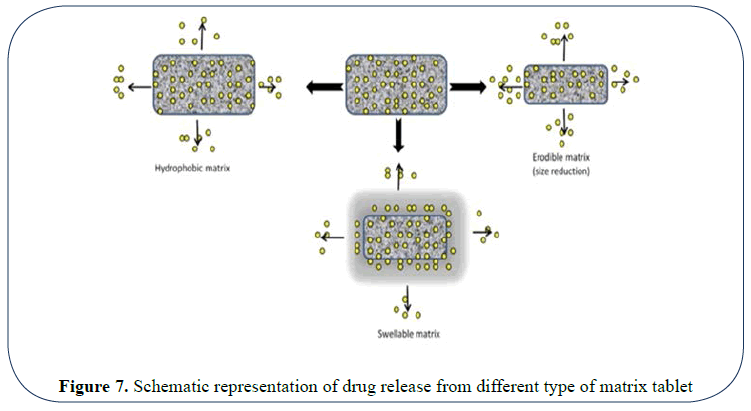
Figure 7: Schematic representation of drug release from different type of matrix tablet
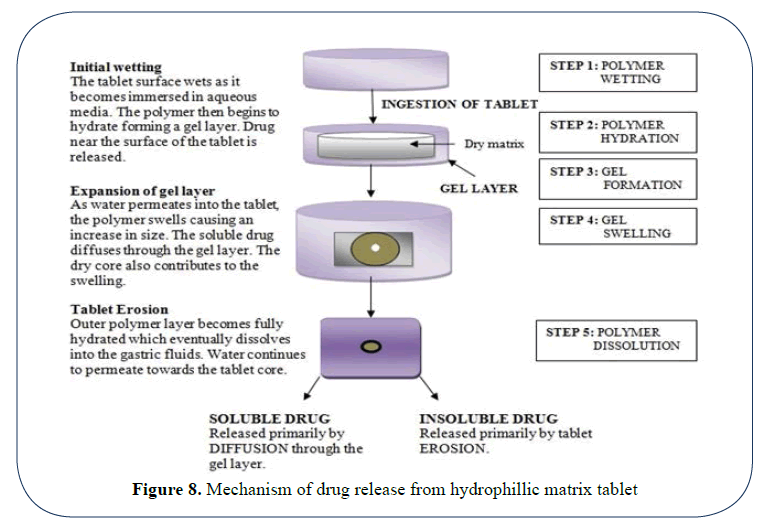
Figure 8: Mechanism of drug release from hydrophillic matrix tablet
Evaluation of Oral Sustained Release Tablets
Thickness of tablet: Thickness of tablet is evaluated by using micrometer screw gauge. Test is carried out randomly on twenty tablets and average values are calculated.
Hardness of tablet: Hardness of tablet of each batch is evaluated by monsantto hardness tester and average values are calculated.
Uniformity of weight: 20 tablets are selected randomly and weighed individually and collectively; average weight is calculated (Table 5).
Table 5.Average weight of tablet showing permissible percentage deviation
| S No. |
Average weight of tablet deviation |
Percentage |
| 1 |
80 mg or less |
10 |
| 2 |
More than 80 mg or less than 250 mg |
7.5 |
| 3 |
250 mg or more |
5 |
% of weight variation=(Individual Weight- Average weight/Average Weight) × 100
Uniformity of content: This test is done to make sure that every tablet should contain the same amount of active ingredient with little or no variation within a batch. For content uniformity test 30 tablets are selected and 10 are assayed individually. At least 9 must assay between ±15% of the declared potency and should not exceed ±25%
Friability: 20 tablets are weighed and placed in fribilator. The chamber is rotated for 4 minutes at a speed of 25 r.p.m. the tablets are removed from the chamber and weighed again. Loss in weight indicates friability. The tablets to be considerd of good quality if loss in weight is less than 0.8%
In vitro dissolution studies: The test is carried out to measure the amount of time required for certain percentage of drug to go into the solution under the specific test conditions. Rotating paddle type and rotating basket type apparatus can be used as per pharmacopoeial standards or as mentioned in monograph of particular drug [42].
The test is passed if for each of the five tablets, the amount of active ingredient in solution is not less than 70% of the stated amount or as specified in the monograph of the API in pharmacopoeia [8,13].
Discussion
The present article is focused on sustained release matrix tablet. The goal of sustained release could be easily and effectively ascertained via approach of matrix tablets. As compared to conventional counterparts matrix tablets offer better patient compliance, maintains constant plasma drug concentration level, reduces chances of toxicity and once a day drug therapy reduces overall cost of treatment. Maintenance of drug concentration within therapeutic range is helpful in minimizing irrational use of drugs (esp. antibiotics) as well as helpful in the treatment of chronic diseases. Furthermore it is a cost effective approach.
Conclusion
It is concluded that, Oral Sustained Release tablets provide the drug release in a modified form than their counterparts. It is an effective to ascertain the therapeutic goals with maximum patient compliance. However, accurate adjustment of various physicochemical parameters is necessary. Matrix tablet is helpful in overcoming the problems associated with conventional dosage form. Apart from various advantages associated with it cost effectiveness and once daily dose are the key benefits associated with it. Due to its key benefits and better patient compliance it can easily lead the market by replacing its counterparts.
References
- Chien YW. Oral drug delivery systems in novel drug delivery pharmaceutical technology. Marcel Dekker Inc. New York. 1992;139-52.
- Qiu Y, Zhang G. Research and development aspects of oral controlled release dosage forms. Handbook of pharmaceutical controlled release technology.1st Indian Ed. Replika press. New York. 2005;465-503.
- Chien YW. Oral drug delivery systems in novel drug delivery pharmaceutical technology. Marcel Dekker Inc. New York. Basel. 1992;152-96.
- Chen X, Wen H, Park K. Challenges and new technologies of oral controlled release. Oral Controlled Release Formulation Design and Drug Delivery:Theory to Practice. 2010;257-77.
- Ali J, Khar RK, Ahuja A. A Textbook of Biopharmaceutics& Pharmacokinetics. Birla Publications Pvt. Ltd. Delhi. 2008;252-72.
- Agarwal G, Kaushik A. Pharmaceutical Technology-II. 1stEd. CBS Publishers, New Delhi. 2012;123-34,174-89.
- Brahmankar DM, Jaiswal SB. Controlled release medication. Biopharmaceutics and Pharmacokinetics- A treatise. 2ndEd. VallabhPrakashan. Delhi. 2009;397-400.
- Zalte HD, Saudagar RB. Review on sustained release matrix tablet. Int J Pharm Biol Sci. 2013;3(4):17-29.
- Ratnaparkhi MP,Gupta JP. Sustained release drug delivery system- An overview. Int J Pharma Res Rev. 2013;2(3):11-21.
- Tapaswi RD, Verma P. Matrix tablets: An approach towards oral extended release drug delivery. Int J Pharma Res Rev. 2013; 2(2):12-24.
- Patel H, Panchal DR, Patel U,et al. Matrix type drug delivery system: A Review. : J Pharm Sci Bio-Sci Res. 2011;1(3):143-51.
- Jamini M, Kothari A. Sustained release matrix type drug delivery system: A review. JDDT. 2012;2(6):142-8.
- Liberman H, Lachman L. The theory and practice of industrial pharmacy. 3rd Ed. Verghese Publication House, Bombay. 1991;171-93.
- https://en.wikipedia.org/wiki/Drug_delivery
- Kumar A, Raj V, RiyazMd, et al.Review on sustained release matrix formulations. International Journal of Pharmacy and Integrated Life Sciences. 2013;1(3):1-14.
- Pundir S, Badola A, Sharma D. Sustained release matrix technology and recent advance in matrix drug delivery system: A review. International Journal of Drug Research and Technology. 2013;3(1):12-20.
- https://health.sbmu.ac.ir/uploads/Remington_Essentials_of_Pharmaceutics_-_Felton,_Linda.pdf
- Chugh I, Seth N, Rana AC, et al.Oral sustain release drug delivery system: An overview. International Research Journal of Pharmacy. 2012;3(5):57-62.
- HadiMd A, Lokeswara VB, Pal N, et al.Formulation and evaluation of sustained release matrix tablets of montelukast sodium. International Journal of Pharmacy. 2012;2(3):574-582.
- Indian Pharmacopoeia Commission. The Indian pharmacopoeia, 6th Ed. Ghaziabad. 2010;187-98.
- Haresh M, ThimmasettyJ, Ratan GN. Formulation development and in-vitro evaluation of sustained release matrix tablets of risperidone.Inventi Impact Pharma Tech.2013;(1):28-34.
- Aulton ME. Pharmaceutics: The science of dosage form design. 2nd Ed. Churchill Living Stone, London. 2005;296-8.
- Wise DL. Handbook of pharmaceutical controlled release technology. 1st Ed. Marcel Dekker, Inc., NewYork. 2005;5-24.
- Vyas SP, Khar RK. Controlled drug delivery concepts and advances. 1st Ed.VallabhPrakashan, New Delhi. 2010;1-12.
- Kamboj S, Gupta GD. Matrix tablets: An important tool for oral controlled-release dosage forms. Pharmaceutical Rev. 2009;7(6):1-9.
- Shargel L, Yu ABC. Modified release drug products. Applied Biopharmaceutics and Pharmacokinetics. 4th ed. McGraw Hill, US. 1999;169-71.
- Joshi J, Bhakuni L, Kumar S. Formulation and evaluation of solid matrix tablets of Repaglinide Der Pharm Sin. 2012;3:598-603.
- Mehta RM. Pharmaceutics. VallabhPrakashan, New Delhi. 2002;258-65.
- Tahara K, Yamamoto K, Nishihata T. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methylcellulose 2910. Journal of Controlled Release. 1995;35(1):59-66.
- Tønnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Development and Industrial Pharmacy. 2002;28(6):621-30.
- Pearnchob N, Siepmann J, Bodmeier R. Pharmaceutical applications of shellac: moisture-protective and taste-masking coatings and extended-release matrix tablets. Drug Development andIndustrial Pharmacy. 2003;29(8):925-38.
- Sungthongjeen S, Pitaksuteepong T, Somsiri A, et al.Studies on pectins as potential hydrogel matrices for controlled-release drug delivery. Drug Development and Industrial Pharmacy. 1999;25(12):1271-6.
- Modi SA, Gaikwad PD, Bankar VH, et al.Sustained release drug delivery system: A review. Int J Pharm Res Dev. 2011;2:147-60.
- Bhardwaj TR, Kanwar M, Lal R, et al.Natural gums and modified natural gums as sustained-release carriers. Drug Development and Industrial Pharmacy. 2000;26(10):1025-38.
- Khan GM. Controlled release oral dosage forms: Some recent advances in matrix type drug delivery systems. The Sciences. 2001;1(5):350-4.
- Talukder R, Fassihi R. Gastroretentive delivery systems: A mini review. Drug Development andIndustrial Pharmacy. 2004;30(10):1019-28.
- Korsmeyer RW, Gurny R, Doelker E, et al.Mechanisms of solute release from porous hydrophilic polymers. International Journal of Pharmaceutics. 1983;15(1):25-35.
- Kumar V, Prajapati SK, Soni GC, et al.Sustained release matrix type drug delivery system: a review. World Journal of Pharmacy and Pharmaceutical Sciences. 2012;1(3):934-60.
- Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. Journal ofControlled Release. 2011;154(1):2-19.
- Nokhodchi A, Raja S, Patel P, et al.The role of oral controlled release matrix tablets in drug delivery systems. BioImpacts. 2012;2(4).
- Gurny R, Doelker E, Peppas NA. Modelling of sustained release of water-soluble drugs from porous, hydrophobic polymers. Biomaterials. 1982;3(1):27-32.
- Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Development and Industrial Pharmacy. 2000;26(7):695-708.