Original Article - (2018) Volume 19, Issue 4
Louise Kuhlmann1,2,3, Jakob L Poulsen1,3, Marianne Køhler4, Henrik H Rasmussen3,4, Peter Vestergaard3,5, Asbjørn M Drewes1,3, Søren S Olesen1,3
1Centre for Pancreatic Diseases, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark
2Department of Internal Medicine, North Denmark Regional Hospital, Hjørring, Denmark
3Department of Clinical Medicine, Aalborg University, Aalborg, Denmark
4Centre for Nutrition and Bowel Disease, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark
5Department of Endocrinology, Aalborg University Hospital, Aalborg, Denmark
Received March 13th, 2018 - Accepted June 06th, 2018
Background Chronic pancreatitis is associated with risk factors that may negatively affect bone metabolism and increase risk of developing osteoporosis and low energy fractures. We evaluated the association of osteoporosis in Danish chronic pancreatitis patients with a number of predefined risk factors besides pancreatic exocrine insufficiency. Methods This was a cross-sectional study of 67 outpatients conducted at a tertiary referral centre. Dual-energy x-ray absorptiometry scan was used to examine bone mineral density for the columnar spine and femoral neck. The primary outcome was to identify risk factors associated with osteoporosis in chronic pancreatitis. Several clinical and demographic parameters, including exocrine pancreatic insufficiency, vitamin-D level, as well as muscle function and strength were analysed for association with bone mineral density. Results The median age of patients was 60 years (IQR 51-68) and 40% were women. The prevalence of osteoporosis was 26.9% in patients compared to 9.1% in Danish citizens (OR 2.4 [95% CI; 1.0-5.7]; P=0.042). Muscle function (Timed Up and Go Test), 25(OH)-Vitamin-D level, and body mass index were independently associated with bone mineral density at the femoral neck (all p<0.001), while diabetes (P=0.03) and exocrine pancreatic insufficiency (P=0.006) were independently associated with bone mineral density in the columnar spine. Conclusion The risk of osteoporosis in chronic pancreatitis outpatients associates with several modifiable risk factors in addition to exocrine pancreatic insufficiency. This information should be implemented in outpatient monitoring strategies to improve bone health and decrease risk of low energy fractures.
Absorptiometry, Photon; Osteoporosis; Pancreatitis; Prevalence; Risk Factors
BMD bone mineral density; BMI body mass index; CP chronic pancreatitis; EPI exocrine pancreatic insufficiency
Chronic pancreatitis (CP) is a progressive, inflammatory disease leading to fibrosis and functional impairment of the pancreatic gland a [1], which ultimately affects the exocrine and endocrine function. Exocrine pancreatic insufficiency (EPI) often leads to maldigestion with ensuing malabsorption of micronutrients such as fat-soluble vitamins including vitamin-D [2]. Osteoporosis is typically caused by an increased stochastic bone remodelling to meet the needs of plasma calcium homeostasis. This will affect the bone strength through loss of bone mass because of trabecular penetration. The bone is thereby left weakened and at increased fracture risk. The calcium homeostasis is influenced by several factors, and some of these, including vitamin-D deficiency, may negatively influence bone metabolism and thereby increase the risk for developing osteoporosis. Another predisposing factor for osteoporosis is inactivity. The body is programmed to remodel bone to strengthen the weight bearing bones and shed “unused” bone. If the bone is overused, microdamage will occur due to remodelling. Likewise, microdamage will also occur when bone is shed due to inactivity. Activity is therefore essential in osteoporosis prophylaxis [3].
In keeping with this, CP patients have several potential risk factors for osteoporosis and population based studies have shown that there is an increased incidence of low energy fractures in CP patients compared to matched control groups [3, 4].
Albeit the increased risk of low energy fractures is well established at the population level, the contributing risk factors and their interaction are still incompletely understood at the individual patient level. Hence, most previous studies have mainly focused on EPI as a risk factor for osteoporosis in the context of CP and there has been a relative disregard of other potential risk factors such as excessive alcohol consumption, low body weight, steroid use, endocrine insufficiency, and muscle strength and function [5, 6, 7, 8, 9, 10, 11].
Based on this lack of information we investigated the association between osteoporosis and several potential risk factors in a population of well-characterized Danish CP outpatients. We hypothesized that a low bone mass density (BMD) would be associated with a number of predefined risk factors including EPI and D-vitamin deficiency. The aim of the study was to identify risk factors associated with decreased BMD.
Study Design, Patients, and Normative Data
This was a cross-sectional study conducted at Centre for Pancreatic Diseases, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Denmark from December 2011 through August 2015. All patients with CP referred to our specialised tertiary centre who had a recent Dual-energy X-ray Absorptiometry (DXA) scan (≤12 months) were included. The diagnosis of CP was based on the Lüneburg criteria and CP was defined as a score ≥4 points [12].
The prevalence of osteoporosis in patients with CP was compared to age and gender-matched population derived normative data from Danish citizens as described earlier by Vestergaard et al. [13].
Study Outcomes
The primary outcome was to identify risk factors associated with a low BMD in chronic pancreatitis.
Data Collection and Risk Factors for low BMD
Predefined risk factors for low BMD (see below), demographics, clinical characteristics, and medication were collected at the patients’ first visit in our outpatient clinic. The M-ANNHEIM classification system was used to categorise the aetiology of CP [14].
A number of risk factors that have previously been associated with a low BMD were collected. These were; gender, age, alcohol consumption [15], smoking [16, 17], vitamin-D levels, and EPI [5, 6, 7, 9, 18, 19], opioid treatment [20], diabetes [21, 22], malnutrition (BMI<18.5 kg/m2) [23, 24], and muscle strength and function assessed by handgrip strength (HGS) and Timed Up and Go Test (TUG) [25].
Alcohol consumption was defined as excessive if it exceeded the Danish Health Authorities’ recommendations of a maximum 7 units of alcohol per week for women and 14 units of alcohol per week for men. Tobacco use was specified as number of cigarette packs per day. Opioid use was arbitrary stratified into three groups; no opioids, opioids use <50 morphine milligram equivalents per day, and opioid use ≥50 morphine milligram equivalents per day.
Anthropometric Assessment
Body weight was measured to nearest 0.1 kg using a digital electronic weight (Seca 701, CE0108, Seca, Birmingham, United Kingdom). Height was measured to nearest 0.1 cm using a wall-mounted stadiometer (Seca 222, CE0123, Seca, Birmingham, United Kingdom). Body mass index (BMI) was calculated as measured body weight in kilograms divided by height in meters squared (kg/m2).
Muscle Strength and Function
The HGS was used to estimate the muscle strength. It was measured to the nearest kg using a hydraulic hand dynamometer (NC70142, North Coast Medical, Arcata, CA, USA). The patient was sitting on a chair with the shoulder neutrally rotated, the elbow bend 90º, the wrist in neutral position, and the dynamometer in second handle position. HGS was measured 3 times with intervals of 10-15 seconds. For statistical analysis, the average HGS of the two hands’ highest recordings was used [25].
The TUG was used to assess the patient’s muscle function and mobility. It measures the time that a person takes to rise from a chair, walk three meters, turn around, walk back to the chair, and sit down. The TUG test provides a composite measure of muscle function, requires both static and dynamic balance, and has been validated in various patient groups [26].
Dual-energy X-ray Absorptiometry
BMD (g/cm2) for the lumbar spine (L1-L4) and right femoral neck were assessed using dual-energy X-ray absorptiometry (Hologic Discovery DXA-scanner, Hologic Inc., Marlborough, MA, USA). BMD was expressed as T-scores, as well as age- and sex-adjusted Z-scores based on the manufacturers reference material. Osteoporosis was defined according to the World Health Organization as a T-score ≤2.5 SD below the young adult mean and osteopenia was defined as a T-score between -1.0 SD and -2.5 SD below the young adult mean [27]. A daily quality control program was employed to ensure scanner reliability and coefficient of variations between days were <1%.
Statistical Analysis
Results are presented as means ±SD unless otherwise stated. Normality was checked through inspection of QQplots. The prevalence of osteoporosis was compared to age and gender matched normative data from a population of Danish citizens [13] and prevalence estimates were reported as population proportions with odds ratios (OR) and 95% confidence intervals (CI). Associations between BMD and risk factors were analysed using univariate and multivariate regression analysis with backward stepwise elimination. Bootstrapping based on 5000 samples was used for internal validation of the multivariate estimates. To aid in interpretation of the retrieved findings, ordinal logistic regression was used to generate probability plots illustrating the probability of having osteoporosis or osteopenia as a function of BMI and TUG. A p-value <0.05 was considered significant. The software package STATA version 14.2 (StataCorp LP, College Station, TX) was used for the statistical analysis.
Patient Characteristics
A total of 67 patients were included. The median age was 60 years (IQR 51-68) and 40 % were women. Table 1 reports demographics and clinical characteristics for all patients.
Prevalence of Osteoporosis and Osteopenia
The prevalence of osteoporosis (combined estimate for femoral neck and lumbar spine) in this patient group was 26.9 % in patients with CP compared to 9.1% in the population of Danish citizens (OR 2.4 [95% CI; 1.0-5.7]; P=0.042). The prevalence of osteoporosis for the femoral neck was 18.5 % in CP compared to 7.3% in the Danish normative population (OR 2.7 [95% CI; 0.9-8.2]; P=0.058); for the columnar spine, the estimates were 16.9% and 4.7 % respectively (OR 4.2 [95% CI; 1.1-15.9]; P=0.022) (Figure 1). The prevalence of osteoporosis for the femoral neck (18.5%) was comparable to that observed for the columnar spine (16.9%) (P=0.82).
The prevalence of osteopenia (combined estimate for femoral neck and lumbar spine) was 50.2 % in patients with CP; no estimates were available for osteopenia from the normative database.
Risk Factors Associated with Bone Mineral Density
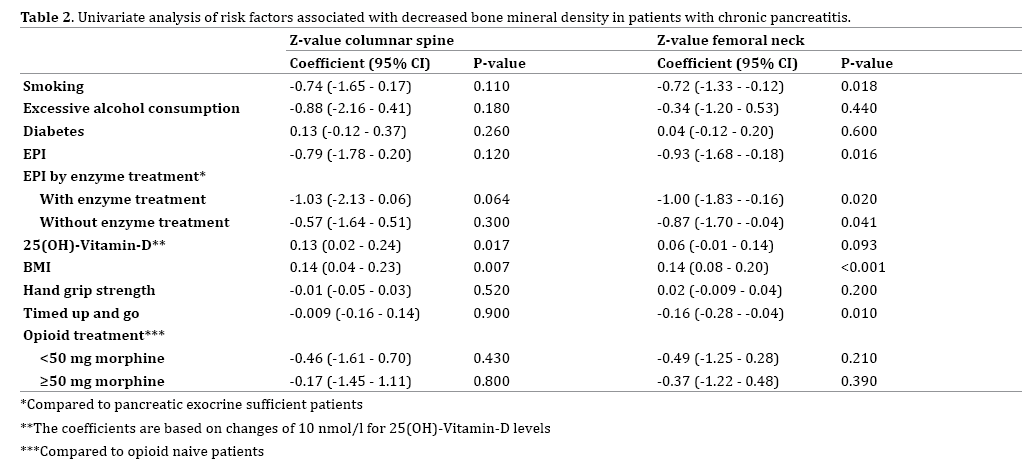
On univariate analysis two risk factors were associated with BMD in the columnar spine; vitamin-D level (coefficient 0.13 g/cm2; P=0.017) and BMI (coefficient 0.14 g/cm2; P=0.007). For the femoral neck four risk factors were associated with BMD on univariate analysis; smoking (coefficient -0.72 g/cm2; P=0.018), EPI (coefficient -0.93 g/ cm2; P=0.016), BMI (coefficient 0.14 g/cm2; P<0.001), and TUG (coefficient (-0.16 g/cm2; P=0.01) (Table 2).
On multivariate analysis, diabetes (coefficient 0.96; P=0.03) and EPI (coefficient -1.07; P=0.006) were independently associated with BMD in the columnar spine, while TUG (coefficient -.17; P<0.001), 25(OH)-Vitamin-D level (0.13; P<0.001), and BMI (coefficient 0.14; P<0.001) were independently associated with BMD at the femoral neck (Table 3).
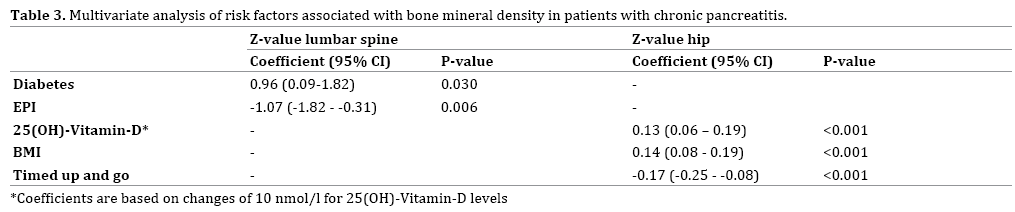
Figure 2a illustrates the risk of osteopenia and osteoporosis as a function of BMI and TUG. A low BMI and a prolonged time to complete the TUG were associated with an increased risk of osteoporosis (Figure 2b).
We investigated the prevalence of osteoporosis and associated risk factors in patients with CP at a tertiary referral centre in Denmark. The patients had an increased risk of osteoporosis compared to an age- and gender matched normative population. Diabetes, EPI, low vitamin-D, low BMI, and impaired muscle function (prolonged Timed Up and Go test) were independently associated with decreased bone mineral density. Our findings underline the importance of systematic evaluation of bone-health in patients with CP even in the absence of EPI.
Prevalence of Osteoporosis
We found a more than twofold increased prevalence of osteoporosis in patients compared to the reference population. Previous studies have reported highly varying prevalence of osteoporosis in CP ranging from 6.7% to 34% [5, 6, 7, 8, 11]. These variations likely reflect differences in study populations including different age and gender distributions, as well as differences in aetiology, severity, duration of CP, and differences in sun exposure. The relatively high prevalence observed in our study may be explained by referral bias as patients were not consecutive and that they were seen at a tertiary centre and thus it is likely that they comprised a cohort of CP patients with more advanced disease stages than observed in primary care or less specialised hospital facilities.
Risk factors for Osteoporosis
Our findings show that different risk factors besides EPI are associated with low BDM. As in several other studies, we found that EPI and low vitamin-D status were associated with a decreased BMD. EPI is present in many patients with CP and in the absence of appropriate enzyme replacement therapy; it may lead to malabsorption of fat and fat-soluble vitamins, including vitamin-D. The latter is an essential hormone for the control of intestinal absorption of calcium and bone mineralization and, as such, vitamin-D deficiency is a well-known risk factor for osteoporosis [28].
Low BMI was independently associated with a decreased BMD in the femoral neck, but not in the lumbar spine. Similar observations have consistently been reported from observational studies and metaanalyses [22, 23, 28, 29, 30, 31]. The pathophysiological mechanisms underlying these observations have not been fully elucidated, but two mechanisms have been proposed: First, individuals with a high BMI conceivable have more adipose tissue than lean individuals and in adipose tissue aromatization of androgens to oestrogens may contribute to maintaining bone health by maintaining a higher level of oestrogens [23]. Second, physical activity and mechanical loading is essential for maintaining a normal bone health and strength [24]. Many studies have investigated the relationship between muscle function, muscle strength, and BMD [32, 33]. Overall, strength training does not affect BMD [32], whereas walking exercises significantly increases BMD in the femoral neck, but not in the lumbar spine [33]. Our findings are in line with these observations; hence, muscle strength was not associated with BMD, whereas muscle function was significantly associated with BMD in the femoral neck, but not the lumbar spine.
Our study showed a positive association between diabetes and BMD in the columnar spine. Previous studies have shown similar results in both type 1 and type 2 diabetes although the fracture risk was found to be higher in these patients than in healthy control subjects [34], indicating that BMD might not be sufficient to evaluate bone quality in these patients.
On univariate analysis, smoking was significantly associated with a low BMD in the femoral neck, but the significance was lost in multivariate modelling. There have been several studies and meta-analysis investigating the relationship between smoking and BMD [16, 17, 34]. Overall studies conclude that smoking is associated with a decrease in BMD of the spine and femoral neck as well as an increased fracture risk. Many studies explain the increased risk of low BMD in smokers with a dose-dependent osteoblast inhibition and/or osteoclast activation from smoking leading to decreased osteogenesis and increased bone resorption [16]. One study hypothesized that the relationship between smoking and decreased BMD was secondary to confounding risk factors, e.g. low BMI which is frequently seen in heavy smokers [17]. This could explain why the association between smoking and BMD in our study was lost in the multivariate model, while BMI remained a significant and independent risk factor for low BMD.
Strengths and Limitations
Strength in the study is that a detailed stratification and analysis of putative risk factors was performed and when using the Z-score for risk factor analysis, we eliminated the confounding effects of age and gender that may have biased previous studies.
Some limitations to this study need to be underscored: First, the patients were not included consecutively, and it is therefore likely that the prevalence will differ from that in the general CP patients. Second, the cross-sectional nature precludes definitive causal inferences about the relationship between osteoporosis and the reported risk factors. However, plausible biological mechanisms exist for all the identified risk factors and these are further substantiated by previous research concerning osteoporosis in other patient groups. Third, the relatively small sample size may introduce a risk of type II errors, which are particularly pertinent for the multivariate analysis and may explain why some risk factors did not reach independent statistical significance. Finally, the study was conducted at a tertiary referral centre, which introduce a risk for selection bias, as the patient`s disease stages in this setting may be more advanced than that observed for the average patient with CP.
To enhance knowledge on the subject, future studies should be performed on larger cohorts with consecutively inclusion.
In patients with CP, low BMI and reduced muscle function were identified as independent risk factors for osteoporosis in addition to EPI and vitamin-D deficiency. These findings underline the relevance of a systematic approach to bone health evaluation in the context of CP even in the absence of EPI. Additionally, focus on modifiable risk factors should be prioritized including, enzyme replacement therapy in the presence of EPI, and maintenance of normal nutritional state, vitamin-D supplementation, and physical activity to preserve muscle function.
Authors declare to have no conflict of interest.