Review Article - (2023) Volume 7, Issue 3
Overview on Gene Therapy Technology
Isayas Asefa Kebede*
Department of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
*Correspondence:
Isayas Asefa Kebede, Department of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo,
Ethiopia,
Email:
Received: 24-Jan-2023, Manuscript No. IPJASLP-23-15553;
Editor assigned: 27-Jan-2023, Pre QC No. IPJASLP-23-15553 (PQ);
Reviewed: 08-Feb-2023, QC No. IPJASLP-23-15553;
Revised: 24-Mar-2023, Manuscript No. IPJASLP-23-15553 (R);
Published:
31-Mar-2023, DOI: 10.36648/2577-0594.7.2.44
Abstract
Gene therapy is a method of prevention or treatment specifically used to treat patients who are suffering from diseases due to defective genes. It is one part of gene based DNA technology and this therapy became possible through advance of genetics and bioengineering that enable manipulation of vector for delivery of gene. It has some requirements, which should be met such as genes of interest must be cloned; treatment should deliver sufficient copies of normal genes to target cells transferred genes should have stable expression. There are two gene therapy types, germ line and the somatic and are two basic delivery systems: In vivo, which involves direct vector injection into the body; and ex vivo, which involves genetic modification of cells in culture followed by transplantation. Gene cannot be directly inserted into an organism’s cell. It must be delivered to the cell using a carrier, or vector. Vector systems can be divided into viral and non-viral. Gene delivery can be used for different purposes. The most common are: functional gene study, cancer therapy, in improving animal production through hormonal therapy like growth hormone and growth hormone releasing hormone therapy, infectious diseases therapies, and so on of the various challenges involved in the process, one of the most significant is the difficulty in releasing the gene into the stem cell which can causes cancer, immune activation and etc. Thus, there should be well established gene therapy research institute to further advancement of their usage.
Keywords
Gene therapy; Gene transfer; Technology; Vector; Growth hormone
Introduction
Gene is a structural, functional and mutational unit of DNA.
Change in natural coding property of a gene is called mutation
which is often lethal. Correcting that mutation is called gene
therapy. This is a technique whereby the absent or faulty gene
is replaced by a working gene, so that the body can make the
correct enzyme or protein and consequently eliminate the
root cause of the disease.
The treatment of gene therapy involves researchers replacing
the defective or faulty genes with a normal functioning gene.
It involves detection of gene, determination of its role, cloning
and introducing the gene by proper way. This is either germ
line gene therapy (done in germ cells) or somatic gene
therapy done in somatic cells [1-3].
Technological advances and the ever growing knowledge of
molecular virology and virus-host cell relationships have
constantly improved the safety profile of viral vectors that are
now used in vitro and in vivo to study cellular gene function,
to correct genetic defects (gene therapy), express therapeutic
proteins, vaccinate against infectious agents and tumors,
produce experimental animal models, and for other purposes. One of the main focuses of this technique is the optimization
of delivery vehicles (vectors) that are mostly plasmids, nano
structured or viruses. The viruses are more often investigated
due to their excellence of invading cells and inserting their
genetic material.
While originally conceived as a way to treat life threatening
disorders (inborn errors, cancers) refractory to conventional
treatment, gene therapy now is considered for many nonlife
threatening conditions, including those adversely affecting a
patient’s quality of life. The lack of suitable treatment has
become a rational basis for extending the scope of gene
therapy.
The potential therapeutic applications of gene therapy are
vast. A major advantage of gene therapy over the use of
conventional drugs is the prospect of curing disease rather
than providing transient relief by suppression of disease
symptoms. Replacing defective genes with functional genes
through the use of gene therapy offers the prospect for long
term therapeutic benefits without repeated drug application
[4-7].
Thus, the objectives of this seminar are:
• To give an overview on types and methods of gene
administration.
• To give highlight on current applications and future
perspective of gene therapy.
• To summarize challenges toward application of gene
therapy.
• To overview viral and physical agent used as gene transfer
vehicle or vector.
Literature Review
Gene Therapy
Gene therapy is a strategy used to treat disease by correcting
defective genes or modifying how genes they are expressed.
The techniques used involve administrating a specific DNA or
RNA sequence. Researchers hope that in the future, gene
therapy will enable patients to be treated by inserting genes
into their cells rather than administering drugs or subjecting
them to surgery. Gene therapy is often aimed at achieving a
long-lasting physiologically matched expression of the gene,
without activating the immune system. The aim is even to
integrate the genetic materials into the chromosome. Gene
therapy promises to revolutionize agriculture as well as
medicine. The early results on the clinical efficiency of gene
therapies were disappointing, largely because the available
gene transfer vectors provoked to be inadequate. Great
progress was made in selecting and improving vectors and
subsequently first positive results were reported.
Gene therapy has some requirements, which should be met.
First of all, genes of interest must be cloned; treatment should
deliver sufficient copies of normal genes to target cells;
transferred genes should have stable expression; modified
cells must have survival advantage over unmodified cells and
finally gene expression must correct or reverse the disease.
Four approaches are now being used in gene therapy. These
are gene augmentation therapy; targeted killing of specific
cell, targeted mutation correction and the last is targeted
inhibition of gene expression [8-11].
Types of Gene Therapy
In general gene therapy can be organized according to its
cellular target, being called somatic gene therapy when the
target is limited to somatic cells. This therapeutic method can
also be considered an ex vivo system, since tissue samples or
cells from the patient must be collected for biopsy with
subsequent reimplantation after the cells are reprogrammed
genetically allowing the correct synthesis of desired gene
product. Another widely used method involves germ cell
lineages generated after collection; the genes of interest are
reprogrammed so that the new features will be perpetuated
for future generations of cells from the patient.
Germ line therapy: This therapy involves the modification of
the genes inside germ cells (sperm or ova). During
reproduction, these gamete cells fuse to form a zygote, which
would divide and pass on the modified gene into all other
cells of the body during the development of offspring. In this
way, the therapy alters the genome of future generations to
come. Although theoretically this could counteract hereditary
disease.
The scientific literature contains over forty reports of the
successful in vitro uptake of exogene constructs (transgenes)
by animal sperm cells. A majority of these reports provide
evidence of post-fertilization transfer and maintenance of
transgenes. Several of the studies report the subsequent
generation of viable progeny animals, the cells of which
contain transgene DNA sequences. While a minority of studies
has used ‘augmentation’ techniques (electroporation or
liposomes) to ‘force’ sperm to capture exogenesis, the
standard methodology is very straightforward: Prior to in vitro fertilization or Artificial Insemination (AI), washed sperm cells
are simply incubated in a DNA containing solution. As a
potential tool for genetically manipulating animals, sperm
mediated gene transfer has the advantages of simplicity and
cost effectiveness, in contrast with more established methods
of transgenes is such as pronuclear microinjection.
Somatic gene therapy: Unlike germ line therapy, somatic gene
therapy only involves the insertion of therapeutic DNA into
body cells and not the germ cells or gametes. This means any
effects of the therapy are confined to the individual being
treated and are not inherited by future offspring.
Several key steps appear to be involved in effective gene
transfer to somatic cells:
• Type of delivery vehicle that may be composed of cationic
liposomes, other types of liposomes, polymers, and their
combinations, various types of viral or hybrid vectors and
combinations of viral vectors with polymers or lipids.
• Interaction of the gene vehicle with serum components.
• Its circulation time in body fluids and bio distribution.
• Its escape from immune cells and macrophages.
• Its interaction with the surface of the cell.
• Its triggering of apoptotic pathways by this interaction.
• Its penetration through the cell membrane barrier.
• Its release from endoscopes or other sub cellular
compartments and its escape from degradation by
intracellular nucleases.
• Nuclear import.
• Ability of regulatory elements for driving the expression of
the foreign gene in a particular cell type including DNA
sequences that might determine integration versus
episomally maintenance of a plasmid or viral vector.
• Persistence of the plasmid in the nucleus (or of the virus)
as an extra chromosomal element for many cell cycles or
integration into active chromatin loci.
• Maintenance of expression for long periods.
• Passage to progeny cells.
• Ability of the transcripts to be exported to the cytoplasm,
translated, modified post-translation ally and transported
through the end plasmatic reticulum and Golgi apparatus
to the cell surface or extracellular [12-15].
Ways of Administration of Gene
There are two basic types of gene delivery systems: In vivo,
which involves direct vector injection into the body; and ex-vivo,
which involves genetic modification of cells in culture
followed by transplantation. Most gene delivery strategies
involve the former. The cells in culture are exposed to the
virus, carrying the desired gene. After infection and
integration of the desired gene in the cell’s DNA, the cells are
returned in the patient by injection into a vein. This technique
is called ex vivo because the gene is transferred to the cells,
while they are outside the patient’s body. In the in vivo technique is the gene of interest is transferred to cells inside
the patient’s body by using of liposomes (fatty particles).
Gene Transfer
Gene cannot be directly inserted into an organism’s cell. It
must be delivered to the cell using a carrier, or vector.
Generally, for full deployment, gene transfer technology
needs to identify the appropriate target, either cell or gene,
and strategy to pursue; deliver the genetic information solely
into the right cell and in the right amount, i.e. selective and
specific targeting and controlled/physiological expression of
the therapeutic gene is a must; maintain the gene and its
expression in the cell long enough to treat the disease or
accomplish the task; restrain the gene from causing short or
long term adverse effects, e.g. triggering autoimmunity,
neoplastic transformation, or other disorders develop delivery
systems with the least possible immunogenicity, and easy to
produce and administer.
Vector systems can be divided into viral vectors and non-viral
vectors. Vectors have several functions, including protecting
the gene from degradation, facilitating entry into target cells,
and securing stable gene transcription upon arrival in the
nucleus. Ideally, a vector should be efficient in gene transfer
and be safe. Safe transfer means that the vector introduces
zero to minimal risk of infection or immunogenicity (immune
response). In addition, a safe vector causes no mutation in the
host cell or patient to patient transmission of a virus or other
pathogens of the viral or non-viral methods of gene transfer,
Retrovirus and Adenovirus based vectors had produced the
best clinical results, but there remained always concern about
the safety of these viruses that were used as vectors in the
delivery of genes. Results also indicated that the somatic cell
gene therapy would be practical and safer approach over
germ line therapy.
Viral vectors for gene transfer: Viral vectors have been used
in >70% of the clinical trials to date. Viruses are vehicles that
efficiently transfer their therapeutic genes. This ability made
them desirable for engineering virus vector systems for the
delivery of therapeutic genes. The viral vectors currently used
in genes into host cells research are based on RNA and DNA
viruses processing. Genomic structures and host ranges.
Particularly viruses were selected as gene delivery vehicles
because of their capacities to carry foreign genes associated
with efficient gene expression. These are the major reasons
why viral vectors derived from retroviruses, adenoviruses,
adeno-associated viruses and herpes viruses are employed in
more than 70% of clinical gene therapy trial worldwide.
Gene transfer technology relies on, and attempts to exploit,
the first step of replication and, at the same time, builds
blocks to prevent production of infectious virus. In this
context, transduction is defined as a non-replicative or dead
end infection that allows heterologous (i.e. non-viral) genetic
information to be delivered recise cell.
The first step of viral vector design is, therefore, to identify
the viral sequences required for replication, assembly of viral
particles, packaging of the viral genome, and delivery of the
transgene into the target cells. Next, dispensable genes are
deleted from the viral genome to reduce replication and
pathogenicity, as well as expression of immunogenic viral
antigens. The gene of interest together with transcriptional
regulatory elements (referred to as the transgene) is inserted
into the vector construct, and a Recombinant virus is
generated by supplying the missing gene products required
for replication and virion production. The more genes that are
removed from the virus, the more replication defective the
vector will be, and there is less chance of recombination to
generate the infectious parental virus. The nature of the virus
biology will usually determine the means of production. For
example, Retroviruses are produced in packaging cell lines,
and vector particles accumulate in the culture medium. In
contrast, Adenovirus and Adeno-Associated Virus (AAV)
vectors are generally produced from transfections, and cells
must be lysed to liberate the viral particles reviews.
Example of gene organization in AAV vector (Figure 1).
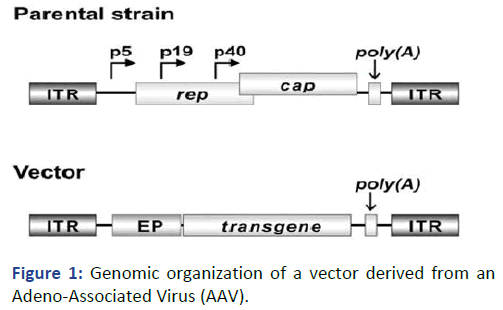
Figure 1: Genomic organization of a vector derived from an
Adeno-Associated Virus (AAV).
The parental genome mostly consists of rep and cap encoding
replicase and structural proteins. Expression of the AAV
proteins is driven by promoter’s p5, p19 and 40 and all
transcripts share the same poly (A). Both poly (A) and
Inverted Terminal Repeats (ITR) are maintained in the vector
genome. By contrast, rep and cap are replaced by the
transgene and the respective Eukaryotic Promoter (EP).
Adeno associated virus vector: Adeno Associated Virus (AAV)
is a small less than 5 kb, single stranded DNA non enveloped
Parvovirus. The natural route of AAV is the upper respiratory
tract. Infection to occur, AAV requires coinfection with Adv
For productive infection which allows the viral genome to
replicate episomally and leads to synthesis of AAV proteins.
The AAV requires an Adenovirus or a Herpes virus for viral
replication. No pathology was linked to this virus.
Adenoviral vectors: Adenoviral vectors are derived from
Adenoviruses (ADV), DNA viruses with a linear double
stranded genome (36 Kilo base pairs, Kbp), a non enveloped
icosahedral capsid with characteristic morphology replicating
in the nucleus and producing thousands of progeny virion
released by cell lysis. The viral genome encodes about 50 viral
proteins, 11 of which are structural and used to physically
build the virion. These viruses have been isolated from a large
number of species, and in humans they primarily infect the
respiratory airways and the gut causing mild and recurrent
respiratory and gastro enteric diseases. Because of their low
pathogenicity, infectious properties, wide tropism, high level
of expression of viral proteins during replication, and the
natural delivery of the viral genome in the nucleus, these
viruses have been considered potential candidates for gene
therapy since its inception [16-19].
Herpes virus vectors: Herpes virus vectors mainly derive from
HSV type-1, a neurotoxic large DNA virus (152 Kbp, double-stranded
DNA) that comprises more than 80 genes
categorized into essential and non-essential genes according
to their requirement for viral replication. In its natural life
cycle, HSV-1 is spread by contact, infects and replicates in skin
membranes, and is taken up by sensory nerve terminals
where it establishes a latent state from which the virus can
subsequently reactivate and spread to other individuals.
These features, high infectivity and ability to transduce and
persist in dividing and non-dividing cells make the HSV vector
a good candidate for gene transfer. The virus contains
essential genes involved in subtle interactions with the host
cell, decoying the immune system, creating conditions for
viral persistence in specific body sites and other functions
that, from the vector point of view, are useless or even
detrimental, and are therefore removed during vector
construction. Removal of non-essential genes makes room for
up to 50 Kb heterologous DNA thus making the HSV vector
the largest carrier among the viral vectors.
Retrovirus vectors: Retroviruses are a group of RNA
containing viruses characterized by the employment of the
unique molecular mechanisms which is an efficient transfer
system. By the reverse transcriptase enzyme activity, the viral
RNA genome to be transcribed into double stranded DNA that
stable integration into host DNA. The advantage of retroviral
vectors are the stable integration into the host genome,
generation of titers that allow efficient gene transfer into a
broad variety of target t cells and the ability to carry foreign
gene up to eighty kb. Vectors based on Lenti viruses such
Human immune deficiency virus have the ability a variety of
post mitotic tissues as heart, muscle or brain, but biosafety
remains a major in production of such vectors. Somatic gene
therapy consists in stable expression of a transgene product
from an implanted group of cells that could be eventually
removed if desired. The vectors used in these cases are
Retroviruses because of their property of integrating into the
transduced cells genome and express the transgene for the
sequential generations in the cell line.
Non-viral vector for gene transfer: Non-viral gene therapy is
the introduction of therapeutic genes via plasmid DNA into
target cells without the use of a virus. In general, these
techniques demonstrate low toxicity, immunogenicity, and
pathogenicity, rendering these techniques safer than viral
methods. However, they demonstrate low transduction
efficiencies, which make them less desirable. These techniques
include microinjection, electroporation, nonoperation, gene
gun, controlled, and chemical delivery methods.
Comparative with viruses, the non-viral techniques for gene
transfer in vivo, the direct injection of plasmid DNA is simple
to use, easy to produce on large scale, inexpensive and safe,
as it lacks specific immune response. In addition to expression
of reporter enzymes, skeletal muscle is now used as a
bioreactor to express therapeutic proteins having either a
local or systemic effect. This methodology was limited by the
relatively low expression levels due to inefficient DNA uptake
into muscle fibers to ensure systemic physiological levels of
secreted proteins.
Naked DNA microinjection: This method involves
microinjection of purified circular DNA into target cells. This has
been mainly performed using mouse skeletal muscle cells. In
relative comparison terms, this method of delivery has shown
to be more efficient when using mouse skeletal muscle tissue
than with adenoviral and retroviral systems; however, the time
course of expression is transient. This delivery system limits its
efficient transfer characteristics only in the skeletal muscle
because the muscle cells have an extensive tubular system,
allowing the DNA delivery. It has been tried in vaccination procedures, because a low and short expression is sufficient
to induce immune response.
In gene gun system, micron-sized gold particles are coated
with plasmid DNA and then accelerated at high speed toward
target cells. Cells penetrated by the gold particles have high
probability of being transfected by the DNA thus introduced.
Micron gold metal particles are used as carrier of plasmid
DNA containing desired gene.
Electroporation (EP) includes cell electro permeabilization,
with the help of exposure to appropriate electric field pulses,
is currently receiving much attention as a way to increase DNA
delivery. In EP, brief electric pulses activate transient pores in
the cell membrane and convey the agents into the cytosol. EP
often does not induce any critical harmful end results, and
therefore various veterinary clinical trials have demonstrated
the safety and efficacy of Electro Chemotherapy (ECT),
chemotherapy delivered via EP. The combination of
cytotoxicity with chemotherapy along with the anti-tumour
immune responses of immune modulatory therapy hinder
tumour growth in multiple types of cancer and
Electroporation (EP) appears as an attainable approach for
cautiously and adequately combining this therapeutics.
Electroporation is therefore a real technique for transfecting
agents, such as chemotherapeutics and plasmid DNA (pDNA),
into host cells. EP is increasingly being used among the
scientific and the medical communities, as it is a safe and
efficient technique to transfer a variety of material (e.g.
nucleic acids, cytotoxicity drugs and ions) into target cells and
tissues without harming them. However, the transfection
efficiency assisted DNA delivery is still low compared to viral
methods and there is a clear need to optimize this approach
[20].
Liposomes: The liposome mediated delivery of genes relies
upon the electrical charge properties of 3 components: The
negatively charged DNA (attributed by the phosphate
backbone of the double helix), the positively charged
liposome, and the net negative charged cell surfaces, owing
to the presence of sialic acid residues. The interplay of these
components results in the liposome-DNA complex fusing with
cell membrane plasmid DNAs degraded in the end lysosomal
pathway, hence very little DNA actually reaches the nucleus.
Nanoparticles: Nanoparticles (NPs) offer an alternative to the
use of viral vectors in gene therapy. NPs are particles that are
1 nm-100 nm in size. There are several different forms of
nanoparticles and they typically contain a segment of DNA or
RNA that is compacted with a polycationic polymer. Due to
their small size, NPs can readily interact with molecules on the
cell surface or inside cells. Unlike their viral vector
counterparts, NPs do not introduce additional genes cells.
They tend to be less immunogenic and cytotoxicity than viral
vectors. Finally, NPs are able to incorporate numerous ligands
such as DNA, antibodies, peptides, and probes and therefore
present an array of therapeutic modalities. Pathways of
cellular internalization of NPs include phagocytosis,
micropinocytosis, clathrin or caveolae mediated endocytosis,
and other clathrin and caveolae independent endocytic
pathways.
Discussion
Current Application and Future Perspectives of Gene
Therapy
Current applications: Gene delivery can be used for different
purposes. The most common are: Functional gene studies,
correction of genetic defects expression of therapeutic
proteins, and immunization against tumors and infectious
agents.
In cancer treatment: Development of high technology
pharmaceuticals against cancer based on gene therapy
systems promise to elicit negligible side effects and to bring a
major advancement and revolution in molecular medicine.
For example, demonstration of tumor regression in animal
xenograft models using virus liposome combination systems
and their feasibility inhuman clinical trials would lead to the
development of novel pharmaceutical products based on
virus liposome complexes; endeavor, although it may appear
to come from a science fiction movie, is feasible and has a
potential in the 60 billion dollar anticancer pharmaceutical
market.
In livestock production: Most important affecting factors are
nutrition and disease. Animal diseases cause great reduction
in their production. Use of gene therapy may significantly
contribute in curing diseases and making animal healthier and
more productive. Some examples of gene therapy are gene
therapy of lysosomal storage diseases.
Growth enhancement: By using naked DNA porcine Growth
Hormone (GH) treatment induces insulin resistance of protein
metabolism and consequently reduces theoretical possibility
for increased protein synthesis in the fed state. GH markedly
reduces the amount of carcass fat; consequently, the quality
of products increases Life tide® SW5 is the world's first and
only approved growth hormone releasing hormone (GHRH)
DNA therapy for food animals. It is an injectable DNA plasmid
encoding for porcine GHRH and administered once for a life
time treatment in sows of breeding age not only to increase
the growth rate but also to increase number of piglets born
alive and weaned. After intramuscular injection and
electroporation, the active constituent Life tide® SW5
plasmid sequence enters to the skeletal muscle cell at the
injection site and resides within the muscle cell. Then the
treated muscle cells actively produce GHRH at physiological
concentrations. The GHRH induces the animal to produce and
secret indigenous growth hormone under the control of
normal physiological feedback mechanism.
In vaccine production: By using plasmid DNA vaccination is
one of the most effective and sustainable methods of
controlling disease. A recent approach has been to use
vaccines based on DNA. The use of DNA in vaccines is based
on the discovery that injecting genes in the form of plasmid
DNA can stimulate an immune response to the respective
gene products. This immune response is a result of the genes
being taken up and expressed by cells in the animal after
injection. Compared to most traditional vaccines, which preferentially elicit a humoral response, immunization by
means of recombinant viral vector also triggers a robust
Cytotoxic T Lymphocyte (CTL) response. That is particularly
efficient in eliminating virus infected cells, intracellular
pathogens, and cancer cells, and extending protection to
other strains of the same pathogen by recognizing highly
conserved epitomes. Vaccine approaches to prevent and treat
prion infection. Molecular basis of pathogenesis of FMDV.
Recombinant Adenovirus co-expressing capsid proteins of two
serotypes of Foot and Mouth Disease Virus (FMDV), in vitro characterization and induction of neutralizing antibodies
against FMDV in swine.
Future perspectives: Since the first clinical gene therapy trial
was conducted, much attention and considerable promise has
been given to the field. There has been substantial public and
private sector investment, as well as increasingly higher levels
of research activity. Numerous preclinical animal model
studies have provided proofs of concept for multiple potential
clinical applications. Also, major advances have been made in
understanding vector biology and improving vector design
and production.
RNA interference or gene silencing may be a new way to treat
Huntington's. Short pieces of double stranded RNA (short,
interfering RNAs or si RNAs) are used by cells to degrade RNA
of a particular sequence. If a si RNA is designed to match the
RNA copied from a faulty gene, then the abnormal protein
product of that gene will not be produced. New gene therapy
approach repairs errors in messenger RNA derived from
defective genes. Technique has potential to treat the blood
disorder thalassemia, cystic fibrosis, and some cancers.
Challenges of Applications of Gene Therapy
Gene delivery: The various challenges involved in the process,
one of the most significant is the difficulty in releasing the
gene into the stem cell. Thus, a molecular carrier called a
“vector” is used to release the gene, which needs to be very
specific, display efficiency in the release of one or more genes
of the sizes necessary for clinical applications, not be
recognized by the immune system and be purified in large
quantities and high concentrations so that it can be produced
and made available on a large scale. Once the vectors inserted
into the patient, it cannot induce allergic reactions or
inflammatory process; it should increase the normal
functions, correct deficiencies, or inhibit deleterious activities.
Furthermore, it should be safe not only for the patient, but
also for the environment and for the professionals who
manipulate it. Finally, the vector should be capable to express
the gene, in general, for the patient’s entire life.
Immune response: Gene delivery vectors must be able to
escape the host’s natural immune surveillance systems. The
immune system may also prevent repeat administration of a
vector, resulting in only short term gene correction. Host
immune responses to vector proteins or to the transferred
gene product (protein) have been a significant obstacle to
achieving therapeutic levels of gene transfer and expression in
some cases notably the hemophilias.
Disrupting important genes in target cells: A gene that is
introduced into any group of cells ideally needs to remain
intact and continue to function inside the cell. For this the
freshly introduced gene must integrate into the cells existing
nuclear DNA which is a random process i.e. not site specific
that may lead to integration of foreign gene within the
existing nuclear genes causing disruption of those genes in
the cells. Integration into the host cell genome has resulted in
insertional mutagenesis and oncogenic transformation in
clinical trials for X-linked severe combined immunodeficiency
disease.
Conclusion
Gene therapy is one of the recent gene based technologies
that is obtaining progressive advance. Although several
protocols have been successful, the gene therapy process
remains complex, and many techniques need new
developments. The specific body cells that need treatment
should be identified and accessible. A way to effectively
distribute the gene copies to the cells must be available, and
the diseases and their strict genetic bonds need to be
completely understood. Gene therapy has been named the
medicine of the future. Besides it considered as it would
revolutionize agriculture by applying effective and safe
methods for animal production through using effective and
efficient disease treatment and prevention methods in
animals and applying hormones that enhance growth and
carcass quality of animal.
Recommendations
Based on above conclusion the following recommendations
are forwarded:
• It is important for government to adopt and develop this
technology to country as it is essential in treatment and
production improvement of animal.
• There should be essential environmental considerations
up on production of vector especially viral vectors.
• There should be well established gene therapy research
institute to further advancement of their usage.
References
- Ana PC, Bruce JB (2008) Gene Therapy: Some history, applications, problems, and prospects. Tox Patho. 36:97-103.
[Crossref] [Google Scholar] [PubMed]
- Bainbridge J, Ali R (2008) Success in sight: The eyes have it! Ocular gene therapy trials for LCA look promising. Gene Ther. 15:1191-1192.
[Crossref] [Google Scholar] [PubMed]
- Bank A (2007) Human somatic cell gene therapy. Bio essays. 18(12):999-1007.
[Crossref] [Google Scholar]
- Bertram J (2000) The molecular biology of cancer. Mol Aspects Med. 21(6):167–223.
[Crossref] [Google Scholar] [PubMed]
- Boulikas T (1998) Status of gene therapy in 1997: Molecular mechanisms, disease targets, and clinical applications. Gene Ther Mol Biol. 1:1-172.
[Google Scholar]
- Cai X, Conley S, Naash M (2008) Nanoparticle applications in ocular. Vision Res. 48(3):319-324.
[Crossref] [Google Scholar] [PubMed]
- Callejas D, Mann C, Ayuso E, Lage R, Grifoll I, et al. (2013) Treatment of diabetes and long term survival following insulin and glucosidase gene therapy. Diabetes. 65. 1-161.
[Google Scholar]
- Calvet C, Mir L (2016) The promising alliance of anti-cancer electro chemotherapy with immunotherapy. Cancer Metastasis Rev. 35:165-177.
[Crossref] [Google Scholar] [PubMed]
- Cavazzana M, Thrasher A, Mavilio F (2004) The future of gene therapy. Nat. 427(6977):779-781.
[Crossref] [Google Scholar] [PubMed]
- Crystal RG (1995) Transfer of genes humans: Early lessons and obstacles to success. Sci. 270:404-410.
[Crossref] [Google Scholar] [PubMed]
- Cutrera J, King G, Jones P, Kicenuik K, Gumpel E, et al. (2015) Safe and effective treatment of spontaneous neoplasms with interleukin 12 electro chemo gene therapy. J Cell Mol Med. 19:664-675.
[Crossref] [Google Scholar] [PubMed]
- Davis H, Demeneix B, Quantin B, Coulombe J, Whalen RG (1993) Plasmid DNA is superior to viral vectors for direct gene transferinto adult mouse skeletal muscle. Hum Gene Ther. 4:733-740.
[Crossref] [Google Scholar] [PubMed]
- Davison A, Benko M, Harrach B (2003) Genetic content and evolution of Adenoviruses. J Gen Virol. 84:2895-2908.
[Crossref] [Google Scholar] [PubMed]
- Delteil M, Golzio C, Dumond C, Draghia P, Teissie J (1998). In vivo electrically media ted protein and gene transfer in murine melanoma. Nat Biotechnol. 1(6):168-171.
[Crossref] [Google Scholar] [PubMed]
- Draghia A, Fiorotto ML, Hill L, Malone PB, Deaver D, et al. (1999) Myogenic expression of an injectable protease resistant growth hormone releasing hormone augments long term growth in pigs. Nat Biotechnol. 17:1179-1183.
[Crossref] [Google Scholar] [PubMed]
- Edelstein M, Abedi M, Wixon J, Edelstein R (2004) Gene therapy clinical trials worldwide 1989-2004 an overview. J Gene Med. 6:597-602.
[Crossref] [Google Scholar] [PubMed]
- Farjo R, Skaggs J, Quiambao A, Cooper M, Naash M (2006) Efficient nonviral gene transfer with compacted DNA nanoparticles. PLoS One. 1(1):38.
[Crossref] [Google Scholar] [PubMed]
- Rives N, Milazzo JP, Perdrix A, Castanet M, Joly-Helas G, et al. The feasibility of fertility preservation in adolescents with Klinefelter syndrome. Hum Reprod. 28(6):1468-1479.
[Crossref] [Google Scholar] [PubMed]
- Fisher K, Jooss J, Alston Y, Yang S, Haecker k, et al. (1997) Recombinant Adeno associated virus for muscle directed gene therapy. Nat Med. 3:306-312.
[Crossref] [Google Scholar] [PubMed]
- Gandolfi F (2000) Sperm mediated transgenesis. Reprod Fertil Dev. 53(1):127-137.
[Crossref] [Google Scholar] [PubMed]
Citation: Kebede IA (2023) Overview on Gene Therapy Technology. J Anim Sci Livest Prod. 7:44.
Copyright: © 2023 Kebede IA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.