- (2006) Volume 7, Issue 4
Muhammad Wasif Saif*
Yale University School of Medicine. New Haven, CT, USA
Despite advances in our understanding of the molecular and genetic basis of pancreatic cancer, the disease remains a clinical challenge. Gemcitabine, the standard chemotherapy for pancreatic cancer, offers modest improvement of tumor-related symptoms and marginal advantage of survival. New approaches, alone and in combination with gemcitabine, are being developed to combat this cancer. Combination chemotherapy trials incorporating gemcitabine, cisplatin, 5- fluorouracil, oxaliplatin, or irinotecan generally show improved outcomes in objective response rates but with little or no improvement in survival in phase III trials. In this article, the author describes the key studies presented at the Annual Meeting of ASCO, held in Atlanta, GA from June 2nd to 6th. The studies discussed here include the following: RTOG 9704 (#4007), FFCDSFRO study (#4008), meta-analysis of gemcitabine plus cisplatin and gemcitabine plus oxaliplatin vs. gemcitabine alone (GERCOR #4003), and ECOG 6201 (Late Breaking Abstract #4004). Based on the results presented at the annual meeting, it comes to us that patients with locally advanced vs. metastatic pancreatic cancer should be studied separately, better understanding of the biology of pancreatic cancer is mandatory and evaluation of novel agents is crucial. We as oncologist have to change our attitudes towards clinical trials and need to think beyond a trial design such as gemcitabine vs. drug of our choice. Environment within which research is being conducted also has to be changed and last but not the least, access to trials for patients with pancreatic cancer is the key step in the fight against pancreatic cancer.
bevacizumab; cetuximab; Cisplatin; Chemotherapy, Adjuvant; gemcitabine; Epidermal Growth Factor; erlotinib; Fluorouracil; oxaliplatin; Pancreatic Neoplasms; Radiation; Radiotherapy, Adjuvant; Quinazolines; Vascular Endothelial Growth Factor A
ASCO: American Society of Clinical Oncology; CONKO: Charité Onkologie - clinical studies in GI cancers; ECOG: Eastern Cooperative Oncology Group; EGFR: epidermal growth factor receptor; ESPAC: European Study Group of Pancreatic Cancer; FA: folinic acid; FDR: fixed dose rate; FFCD-SFRO: Federation Francophone de Cancerologie Digestive and Societe Francaise de Radiotherapie Oncologique; Gem: gemcitabine; GemOx: gemcitabine plus oxaliplatin; GERCOR: Groupe d'Etude et de Recherche en Cancreologie Onco-Radiotherapic; GISCAD: Italian Group for the Study of Gastrointestinal Tract Carcinomas; GITSG: Gastro-Intestinal Study Group; HR: hazard ratio; LBA: late breaking abstract; PS: performance status; RECIST: Response Evaluation Criteria in Solid Tumors; RTOG: Radiation Therapy Oncology Group; XRT: radiation/ radiotherapy
Despite advances in our understanding of the molecular and genetic basis of pancreatic cancer, the disease remains a clinical challenge. Gemcitabine, the standard chemotherapy for pancreatic cancer, offers modest improvement of tumor-related symptoms and marginal advantage of survival. New approaches, alone and in combination with gemcitabine, are being developed to combat this cancer. Combination chemotherapy trials incorporating gemcitabine, cisplatin, 5- fluorouracil, oxaliplatin, or irinotecan generally show improved outcomes in objective response rates but with little or no improvement in survival in phase III trials. In this article, the author describes the key studies presented at the Annual Meeting of ASCO, held in Atlanta, GA from June 2nd to 6th. The studies discussed here include the following: RTOG 9704 (#4007), FFCDSFRO study (#4008), meta-analysis of gemcitabine plus cisplatin and gemcitabine plus oxaliplatin vs. gemcitabine alone (GERCOR #4003), and ECOG 6201 (Late Breaking Abstract #4004). Based on the results presented at the annual meeting, it comes to us that patients with locally advanced vs. metastatic pancreatic cancer should be studied separately, better understanding of the biology of pancreatic cancer is mandatory and evaluation of novel agents is crucial. We as oncologist have to change our attitudes towards clinical trials and need to think beyond a trial design such as gemcitabine vs. drug of our choice. Environment within which research is being conducted also has to be changed and last but not the least, access to trials for patients with pancreatic cancer is the key step in the fight against pancreatic cancer.

Adenocarcinoma of the pancreas is the fourth leading cause of cancer death in the United States. According to the American Cancer Society, the 1-year relative survival rate is only 20% and 5-year survival only 4% for all stages combined. Over the years, a number of chemotherapy doublets have been evaluated without significantly improving survival, thus leaving single-agent gemcitabine as the standard of care for the treatment of this disease.
Despite advances in our understanding of the molecular and genetic basis of pancreatic cancer, the disease remains a clinical challenge. Gemcitabine, the standard chemotherapy for pancreatic cancer, offers modest improvement of tumor-related symptoms and marginal advantage of survival. New approaches, alone and in combination with gemcitabine, are being developed to combat this cancer. In this article, the author describes the key studies presented at the annual meeting of ASCO, held in Atlanta, GA from June 2nd to 6th.



Staley’s classification offers a simple model for groups engaged in protocol-based clinical research examining innovative multimodality treatment strategies for patients with pancreatic cancer [1].
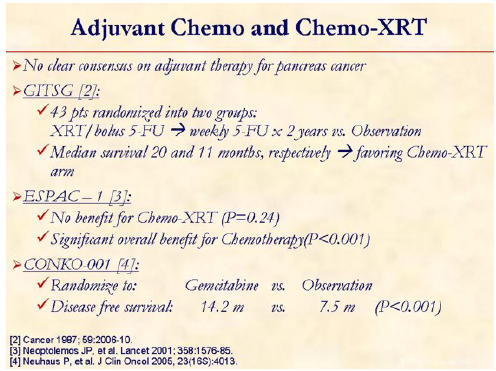
There is no consensus on what constitutes ‘standard’ adjuvant therapy. The high rate of locoregional failure following surgical resection for adenocarcinoma of the pancreas has prompted investigators to evaluate the role of adjuvant chemo-XRT. The Gastrointestinal Tumor Study Group (GITSG) [2] showed improved survival in the patients receiving adjuvant chemo-XRT (21 months) vs. observation (10.9 months) and set up the platform for future studies. The European Study Group for Pancreatic Cancer (ESPAC) assessed the roles of chemo-XRT and chemotherapy in a randomized study: ESPAC-1 [3]. The median survival for patients receiving chemo-XRT was 15.5 months, compared with 16.1 months among patients who did not receive chemo-XRT (HR: 1.18, 95% CI: 0.90-1.55; P=0.24). The median survival for patients receiving chemotherapy was 19.7 months, compared with 14.0 months in patients who did not receive chemotherapy (HR: 0.66; 95% CI: 0.52-0.83; P<0.001). Interpretation of this study is complicated slightly because 2 different study designs are used: a 2x2 factorial design and direct head-to-head comparisons (chemotherapy vs. no chemotherapy and chemo-XRT vs. no chemo-XRT). Eligible patients were pre-enrolled in one of the above strategies. The authors then reported their findings for each of the separate study designs as well as for the pooled data. The question is whether this study should change our practice with regard to how we treat patients whose pancreatic cancer was resected. The answer is no - at least not yet. XRT, at the very least, serves to decrease the chances of local recurrence (not examined in this study), which ultimately may influence patients' quality of life down the road. However, a compelling argument can be made that identification of an effective systemic regimen to eradicate micrometastases and reduce the opportunity for metastasis may not be the most critical factor in improving these patients' chances for longterm survival. This ESPAC-1 study uses only a 5-FU-based chemotherapy regimen; and certainly, a gemcitabine-based approach is the most logical place to start, which was recently evaluated in combination with chemo-XRT (using 5-FU as radiosensitizer) in the RTOG 9704 study presented at the annual meeting of ASCO, 2006. Moreover, CONKO-001 study compared gemcitabine vs. observation [4].
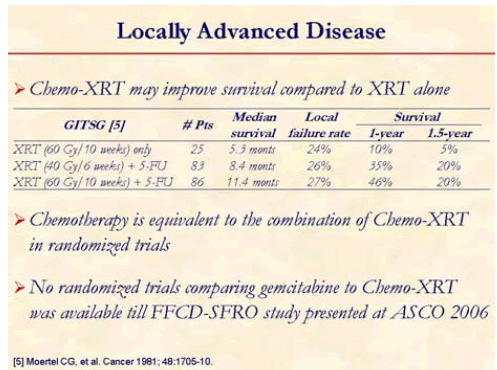
For patients with localized disease that is not amenable to surgical resection, chemotherapy and radiotherapy or chemotherapy are the common treatment options. The addition of chemotherapy to radiation may enhance the local effects of radiation or provide treatment of disease outside the radiation field. The results of clinical trials evaluating the appropriate therapy for locally advanced or resected disease have been inconsistent. Recognizing which patients are likely to benefit from combination therapy or systemic therapy alone is a subject of future and ongoing clinical trials [5].
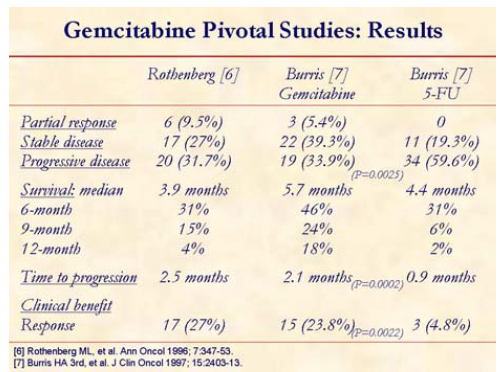
Gemcitabine, the standard chemotherapy for pancreatic cancer, offers modest improvement of tumor-related symptoms (clinical benefit response) and marginal advantage of survival [6, 7].
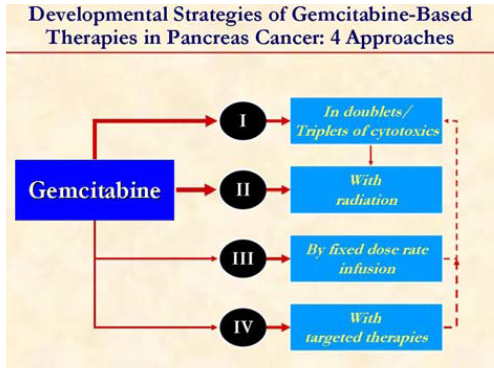
Strategies to improve the efficacy of gemcitabine include combining with other cytotoxic agents, biologic agents, or radiation or administer as a FDR infusion.

On the basis of pharmacokinetic data, studies have been performed using an FDR of gemcitabine of 10 mg/m2/min in an effort to maintain a critical plasma concentration of gemcitabine, and thus increase tumor cytotoxicity and therapeutic efficacy.
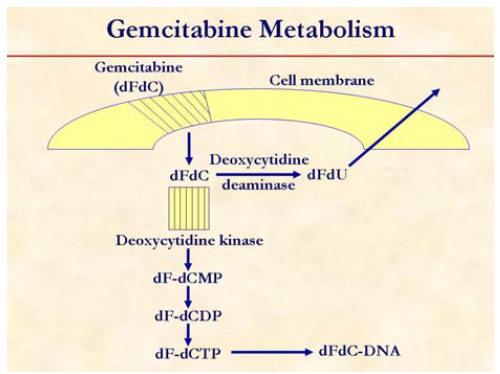
Gemcitabine is a prodrug that is initially phosphorylated by deoxycytidine kinase to gemcitabine monophosphate (dF-dCMP), and subsequent phosphorylation steps yield gemcitabine diphosphate (dF-dCDP) and gemcitabine triphosphate (dF-dCTP). Gemcitabine diphosphate inhibits ribonucleotide reductase, decreasing the cellular pool of deoxycytidine triphosphate that competes with gemcitabine triphosphate for incorporation into DNA. Incorporation of gemcitabine triphosphate into DNA inhibits replication with subsequent induction of apoptosis. Gemcitabine is cleared through metabolic elimination by cytidine deaminase and cytidylate deaminase, respectively. Phosphorylation of gemcitabine to the monophosphate by deoxycytidine kinase is the rate-limiting step in the accumulation of the active diphosphate and triphosphate metabolites. The activity of gemcitabine is dependent on its phosphorylation to its triphosphate, the major intracellular metabolite. Although doses of gemcitabine ranging between 800 and 2,800 mg/m2 are generally administered by intravenous infusion over 30 minutes, there is evidence that this generates plasma gemcitabine concentrations that greatly exceed the levels (15 to 20 μmol/L) that saturate the rate of triphosphate accumulation. Alternatively, gemcitabine infusion at the fixed dose rate of 10 mg/m2/min has been demonstrated to maximize the rate of triphosphate formation, and enhance cytotoxicity.

This slide shows the efficacy of the different schedules of gemcitabine used and GemOx in advanced pancreatic cancer (randomized trial) [7, 8, 9].


The studies discussed here include the present ones [10, 11,12, 13].
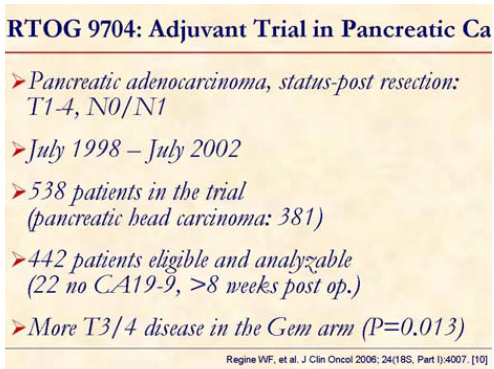
Patients post gross total resection of pancreatic adenocarcinoma were eligible. Patients were stratified by nodal status (uninvolved vs. involved), primary tumor diameter (less than 3 cm vs. equal to or greater than 3 cm) and surgical margins (negative vs. positive vs. unknown).
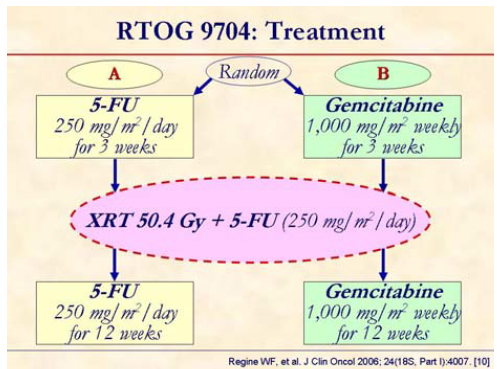
Patients were randomized to receive pre and post chemo-XRT 5-FU vs. pre and post chemo-XRT gemcitabine.
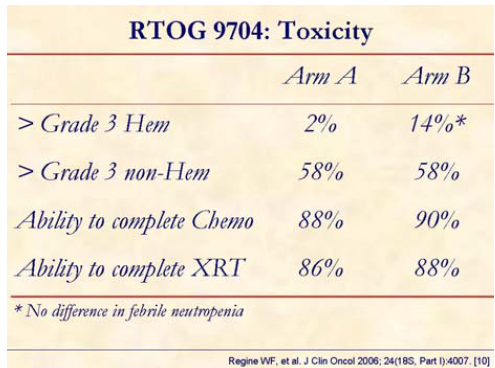
No significant difference in non-hematologic grade equal to or greater than 3 toxicity was seen. The grade 4 hematologic toxicity rate was 14% in the gemcitabine arm and 2% in the 5-FU arm (P<0.001) without difference in febrile neutropenia.
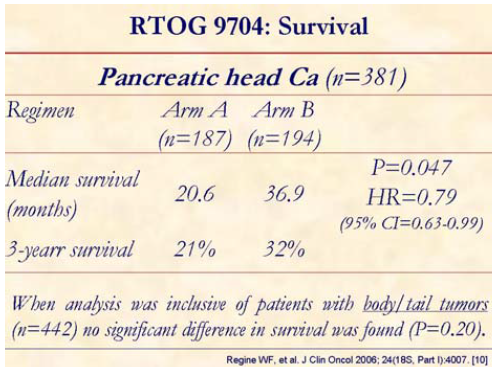
Patients with pancreatic head tumors (n=381) experienced significantly improved survival, with median and 3-year survival of 36.9 months and 32%, respectively, for the gemcitabine arm (B) vs. 20.6 months and 21% for the 5-FU arm (A). When analysis was inclusive of patients with body/tail tumors (n=442) no significant difference in survival was found.
The study concluded that the addition of gemcitabine to postoperative adjuvant 5-FUXRT significantly improves survival in patients with pancreatic head adenocarcinoma.
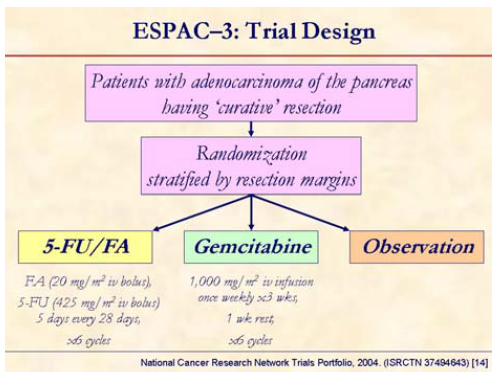
ESPAC-3 (a randomized phase III trial) is currently enrolling patients with resected pancreatic cancer to compare among 5-FU + folinic acid (FA) vs. gemcitabine vs. observation [14].

This randomized study evaluated whether initial Chemo-XRT adds to modern gemcitabine in term of overall survival [5, 7, 15].
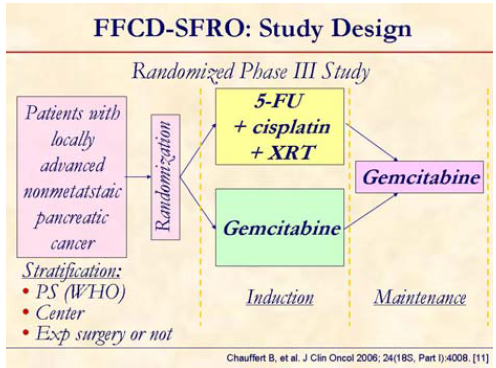
Patients (WHO status 0-2) with confirmed locally advanced, unresectable but nonmetastatic pancreatic adenocarcinoma, were randomized 1:1 between Chemo-XRT (60 Gy in 6 weeks, 2 Gy/fraction, concomitant with 5-FU, 300 mg/m2/day as a continuous infusion, day 1-5 every week and cisplatin, 20 mg/m2/day, day 1-5 at week 1 and 5) or gemcitabine (1,000 mg/m2 weekly for 7 out of 8 weeks) as induction treatment. Maintenance treatment consisted of gemcitabine administered as 1,000 mg/m2 weekly for 3 out of 4 weeks in both arms until progression or limiting toxicity.
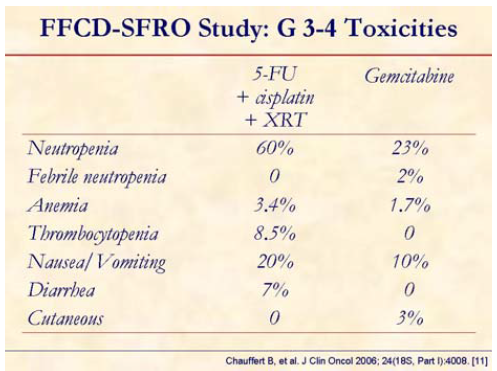
Increased hematological and gastrointestinal toxicity was observed in patients receiving Chemo-XRT.
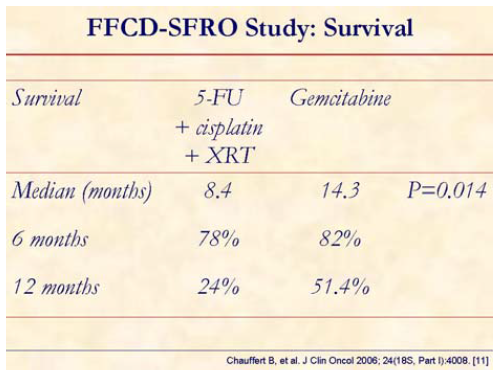
At median follow-up of 16 months, overall survival at 6 and 12 months were 78% vs. 82% and 24% vs. 51%, with a median survival of 8.4 vs. 14.3 months (stratified logrank P=0.014) for chemo-XRT vs. gemcitabine arms, respectively.

The study concluded that gemcitabine alone allowed a significant overall survival in locally advanced nonmetastatic pancreatic cancer. Study was stopped before the planned inclusion due to lower survival with initial chemo-XRT when compared to gemcitabine alone.
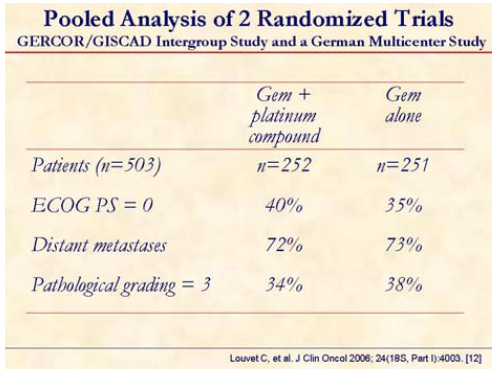
Pooled analysis of two randomized trials (GERCOR/GISCAD: GemOx vs. gemcitabine; German multicenter trial: gemcitabine plus cisplatin vs. gemcitabine) was presented at the meeting. Standard methods for meta-analysis based on individual patient data were used.
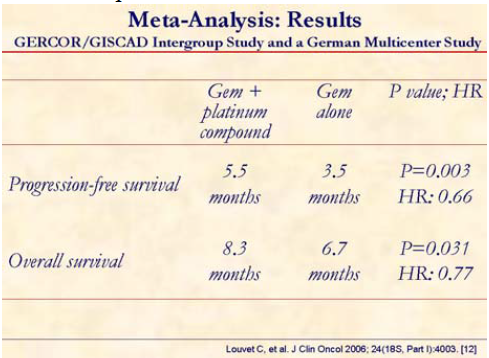
This meta-analysis clearly shows that progression-free and overall survivals were significantly superior in the gemcitabine plus platinum compound patients. In fact, this group of patients had both hazard rates (HRs) significantly lower than 1 when compared to the gemcitabine alone treated group.
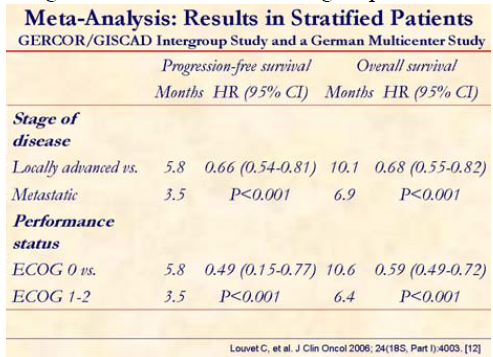
Locally advanced and PS 0 patients may achieve a greater benefit in progression-free, as well as in overall survival.
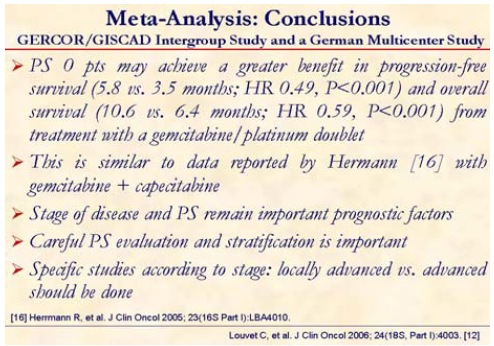
This pooled data analysis concluded that combination of gemcitabine with a platinum analog such as oxaliplatin or cisplatin significantly improves progression-free survival and overall survival as compared to single-agent gemcitabine in advanced pancreatic cancer. PS 0 patients may achieve a greater benefit in progression-free as well as in overall survival. This is similar to data reported by Hermann at the 2005 ASCO Meeting [16] with gemcitabine + capecitabine.
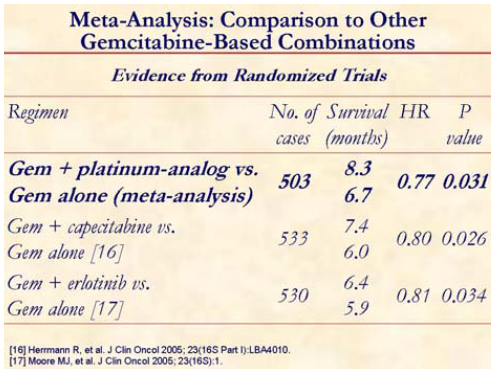
If we compare the benefit of adding a platinum compound with capecitabine or erlotinib from other randomized trials [16, 17], it is evident that a gemcitabine plus platinum agent has a comparable activity.
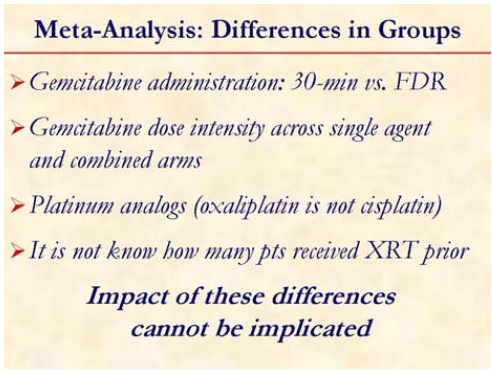
It is important to appreciate that the dose intensity as well as the schedule of gemcitabine was different among the patients included in the study. Also, the platinum agent were different in different studies: oxaliplatin vs. cisplatin and whether these agents are cross-resistant in this disease is not known.
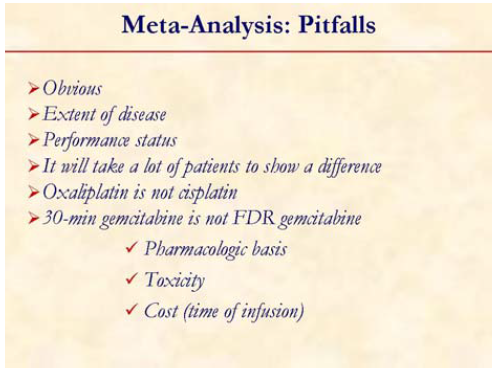
However, the limitations of a pooled analysis cannot be ignored. It is important to note that extent of disease and PS are two important prognostic factors. It will take a lot of patients to show a difference in a randomized trial.

ECOG 6201 compares overall survival of standard gemcitabine 1,000 mg/m2/30-min weekly for 7 out of 8 weeks, and then weekly for 3 out of 4 weeks (arm A) vs. FDR gemcitabine 1,500 mg/m2/150 min (at a rate of 10 mg/m2/min) weekly for 3 out of 4 weeks (arm B) or gemcitabine 1,000 mg/m2/100-min day 1 plus oxaliplatin 100 mg/m2 day 2 every 14 days (arm C).

The primary endpoint of the study is overall survival and secondary endpoints are the comparison of the experimental regimens, toxicity, response, patterns of failure, progression-free survival and quality-of-life.

Prior adjuvant radiosensitizing 5-FU was permitted. Patients were stratified by PS 0-1 vs. 2 and locally advanced vs. metastatic disease.
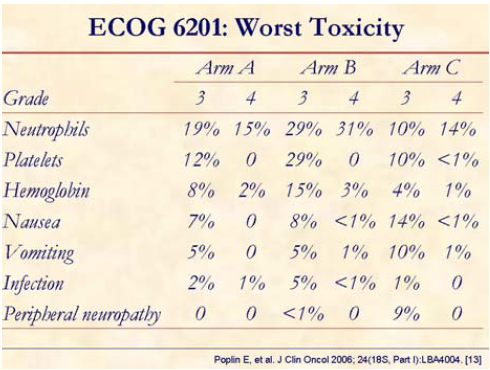
Fixed dose rate and GemOx with increased but manageable toxicity:
• higher hematologic toxicity and nausea and vomiting with fixed dose rate;
• higher neuropathy with GemOx.

GemOx and FDR gemcitabine have higher response rate than 30-minute gemcitabine.
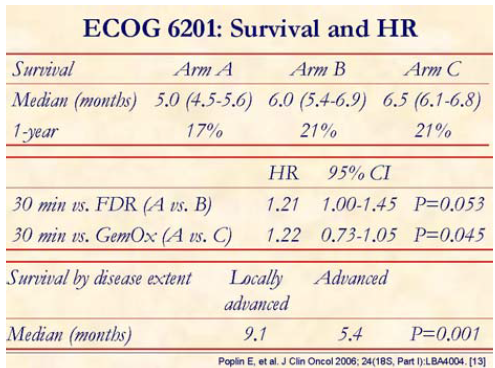
Median overall survival for arms A, B, and C are 4.9, 6.0, and 6.5 months, respectively. Hazard ratio A vs. B is 1.21 with stratified log rank P=0.053 and for A vs. C is 1.22 with stratified log rank P=0.045. Therefore, the overall survival was significantly improved in arm C than in 30-min gemcitabine (arm A).

The study concluded that both FDR and GemOx had approximately 1-month longer median overall survival than standard gemcitabine, but not statistically significant.


Three major randomized studies are evaluating the role of incorporating bevacizumab and cetuximab with gemcitabine and irinotecan plus docetaxel in advanced pancreatic cancer [18, 19, 20].


Based on the results presented at the annual meeting, it again comes to us that a better understanding of the biology of pancreatic cancer is mandatory and evaluation of novel agents is crucial. Newer imaging techniques may help to gauge disease better. Palliative care is an integral part in the management of patients with pancreatic cancer. We can not underestimate this as data suggest that improved pain control alone impacts on survival in this disease.
We as oncologist have to change our attitudes towards clinical trials and need to think beyond a trial design such as gemcitabine vs. gemcitabine plus drug A.


Study design such as locally advanced and metastatic patients need to be studied separately. Environment within which research is being conducted also has to be changed and last, but not the least, access to trials for patients is the key step in the fight against pancreatic cancer.

Single agent gemcitabine remains the standard of care in North America. FDR gemcitabine is not 30-minute infusion gemcitabine. However, the toxicity and cost (time of infusion) associated with FDR gemcitabine cannot be overlooked. Sadly to say, but the further evaluating the role of platinum compounds is not indicated anymore. FCCD-SFRO is another study negating benefit of platinum compounds. However, it is clear that addition of these compounds to gemcitabine offer higher response rate and should not be forgotten. We need to identify surrogates for survival and accelerate testing new drugs, including targeted agents. We must consider focusing on improving adjuvant treatment and secondline treatment as most first-line regimens failed in the last decade. It is also important to standardize our approach towards design, analysis, and reporting. Finally, we need to move away from “ONE SIZE FITS ALL” approach to TAILORED patient management.
The author is grateful to the authors/presenters of the major studies discussed in this review article:
Chauffert B. Unite INSERM 517, Faculty of Medicine, 7, Boulevard Jeanne d'Arc, BP 87900, 21079, Dijon, France. bchauffert@dijon.fnclcc.fr (FCCD-SFRO).
Louvet C. Institut National de la Sante et de la Recherche Medicale (INSERM), Unit 643 and Institut de Transplantation et de Recherche en Transplantation (ITERT), Nantes, France. christophe.louvet@sat.aphop- paris.fr (meta-analysis: GERCOR/ GISCAD).
Poplin EA. Cancer Center of New Jersey, New Brunswick, New Jersey 08901, USA. poplinea@umdnj.edu (ECOG 6201).
Regine WF. University of Maryland School of Medicine, Radiation Oncology Clinical, Gudelsky Tower; Room GGK-17E, Baltimore, MD 21201, USA. wregine@umm.edu (RTOG 9704).