- (2011) Volume 12, Issue 4
Muhammad Wasif Saif
Columbia University College of Physicians and Surgeons. New York, NY, USA
Pancreatic cancer still is a significant, unresolved therapeutic challenge with nearly similar incidence and mortality rates. It is the most lethal type of digestive cancer with a 5-year survival rate of 5%. Adjuvant chemotherapy remains to be gemcitabine alone or combined with infusional 5-fluorouracil with radiation therapy. Nevertheless, only a few patients survive for at least 5 years after R0 resection and adjuvant therapy. Most patients need palliative treatment. Once pancreatic cancer becomes metastatic, it is uniformly fatal with an overall survival of typically 6 months from diagnosis. Chemotherapy is an important component of palliative care but must be administered as a part of a multidisciplinary approach, including palliation of pain, managing weight loss, and deterioration in functional status. Gemcitabine has been the standard in both locally advanced and metastatic disease. The addition of the tyrosine kinase inhibitor erlotinib prolongs median survival for only 2 weeks. While gemcitabine-based regimens are currently accepted as the standard first-line treatment of patients with locally advanced or metastatic pancreatic adenocarcinoma, there is no consensus regarding treatment in the second-line setting. It will not be untrue to say that there are no real medical breakthroughs with regards to improving the prognosis of pancreatic cancer as of 2011. On the other hand, we have made some progress in patients with advanced pancreatic neuroendocrine tumors. These patients have a 5-year survival that can range from 97% in benign insulinomas to as low as 30% in non-functional metastatic pancreatic neuroendocrine tumors. Treatment options may include surgery, transarterial chemoembolization of liver metastases, and cytotoxic therapy such as streptozotocin, 5-fluorouracil or doxorubicin. Somatostatin analogues, like octreotide, have been proven to prolong progression-free survival in patients with metastatic neuroendocrine tumors of midgut origin. In 2011, two targeted agents, a tyrosine kinase inhibitor sunitinib and mTOR inhibitor everolimus have been approved by FDA for pancreatic neuroendocrine tumors. With these approvals, U.S. physicians can now offer their patients with progressive pancreatic neuroendocrine tumors. Patients with any stage of pancreatic cancer should be considered candidates for clinical trials.
Drug Therapy; erlotinib; gemcitabine; Pancreatic Neoplasms
CONKO: Charité Onkologie; FOLFIRINOX: 5- fluorouracil, leucovorin, irinotecan and oxaliplatin
Pancreatic cancers can arise from the exocrine and endocrine parts of the pancreas. Approximately 95% of them develop from the exocrine portion, including the ductal epithelium, connective tissue, acinar cells, and lymphatic tissue. Broadly speaking, there are three basic types: ductal adenocarcinoma (more than 90% of pancreatic cancers); neuroendocrine tumors (rare) and cystic neoplasm (less than 1% of pancreatic cancers). Approximately 75% of all pancreatic carcinomas are located in the head or neck of the pancreas, 15-20% in the body of the pancreas, and 5-10% occur in the tail.
Pancreatic ductal adenocarcinoma accounts for 90% of cancers of the pancreas. In 2010, there were an estimated 43,140 new cases and 36,800 deaths from pancreatic cancer in the United States [1]. This represents the 10th most common cancer diagnosis but the 4th most common cause of cancer-related death among men and women (6% of all cancer-related deaths), highlighting the disproportionate mortality associated with this diagnosis [2].
Resectable Pancreatic Cancer
The only potentially curative therapy for pancreatic cancer is surgical resection. Unfortunately, only 20% patients are resectable at the time of diagnosis [3]. Pancreatic cancer is resectable if the tumor is confined to the pancreas without the encasement of adjacent surrounding major vessels (superior mesenteric artery or vein, portosplenic confluence, celiac trunk, or aorta), or distant metastases. Even among those patients who undergo resection for pancreatic cancer and have tumor-free margins, the 5-year survival rate after resection is 10% to 25% [3]. Because the only potential cure is through surgery, all patients with potentially resectable lesions by CT criteria should be referred for surgical consultation.
No consensus exists on what defines “standard” adjuvant therapy for pancreatic cancer. This controversy derives from several studies, each pregnant with its own limitations. Standards of adjuvant therapy for pancreatic cancer also vary on the geography as chemo-radiotherapy followed by chemotherapy or vice versa is considered the optimal therapy in North America based on the Gastrointestinal Tumor Study Group (GITSG), European Organization of Research and Treatment of Cancer (EORTC), and Radiation Therapy Oncology Group (RTOG)-9704 studies while chemotherapy alone is considered the standard therapy in Europe supported by the European Study Group for Pancreatic Cancer (ESPAC)-1, ESPAC-3, and Charité Onkologie (CONKO) studies [4, 5, 6, 7, 8, 9]. The high rate of locoregional failure following surgical resection for adenocarcinoma of the pancreas has made it clear that some form of adjuvant therapy should be considered in these patients.
Unresectable
Locally advanced pancreatic cancer is defined as the tumor that has not metastasized but encases the celiac axis or superior mesenteric artery, and represents about 25% of pancreatic cancer cases at presentation [10]. Patients with limited vascular involvement by tumor are considered to have borderline resectable disease and are often treated as locally advanced pancreatic cancer. It is widely accepted that a pancreatic tumor is unresectable when distant metastases are present or there is a local invasion or arterial (celiac trunk, hepatic artery, superior mesenteric artery) or venous (portal vein, superior mesenteric vein) vessels. But reality at the time of surgery may be more complex, and a tumor with no vascular invasion may be found to be nonresectable because of desmoplastic reaction. Expertise of surgeons in radical and revascularization techniques may significantly influence tumor resectability.
Although chemotherapy and radiotherapy are not curative, they may offer some clinical benefits, including shrinkage of the primary tumor, improvement of symptoms, and prolongation of survival. Other options of treatment may include chemotherapy alone to induction chemotherapy followed by chemoradiation. The median survival is limited to 10-12 months, leaving significant room for improvement [10]. The patients with unresectable pancreatic cancer should be considered for inclusion into investigational trials.
Advanced/Metastatic
Gemcitabine, with or without erlotinib, has been the standard chemotherapy in this setting but the benefit is only modest [11, 12]. Because gemcitabine has been considered a standard treatment for advanced pancreatic cancer for the past decade, several randomized trials have tested the combination of gemcitabine plus a second agent, including platinum based agents, topoisomerase inhibitors, taxanes, bevacizumab and cetuximab, as biologically “targeted” agents (Figure 1) [13, 14, 15, 16, 17]. Thus far gemcitabine and erlotinib combination is the only combination therapy in pancreatic cancer to ever demonstrate statistically significant benefits in overall survival, but with modest clinical benefit. Randomized studies of other targeted agents (bevacizumab and cetuximab) have been disappointing.
Recently, a randomized phase III study compared gemcitabine versus 5-fluorouracil plus leucovorin plus irinotecan plus oxaliplatin (FOLFIRINOX) (Figure 2). All of the study endpoints favored FOLFIRINOX: median overall survival (11.1 vs. 6.8 months), median progression-free survival (6.4 vs. 3.3 months) and response rates (31.6% vs. 9.4%). Incidences of grade 3 or 4 neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy, as well as grade 2 alopecia, were significantly higher in the FOLFIRINOX arm. This is the first study to show substantial improvements in survival in advanced pancreatic cancer [18].
However, it is important to keep in mind this gain occurs with an aggressive multichemotherapy regimen rather than with the addition of targeted therapy as many had hoped for. The efficacy of the regimen is substantial; concerns about toxicity are substantial too. Anecdotally, many oncologists are empirically reducing the doses of this regimen, in particular irinotecan; however, impact on efficacy cannot be assessed and future studies are required to further evaluate the modification in a prospective manner. The use of growth factor support should also reduce the risk for febrile neutropenia. Careful patient selection is extremely important. Only younger and in excellent performance status patients who wish to seek more aggressive treatment should be considered appropriate candidates for FOLFIRINOX.
Pancreatic cancer persists as a major therapeutic challenge largely characterized by chemotherapyrefractory disease and poor responses to currently available treatments. Possible reasons for pancreatic tumor resistance to targeted agents may include:
• complexity and redundancy of signaling:
– single targeted agent less likely to be effective;
• surrounding desmoplasia:
– role of supporting connective tissue elements (?);
• pancreatic cancer stem cells:
– highly tumorigenic, can generate phenotypic diversity within the tumor;
– may be resistant to standard therapies.
Current data set on treatment options in second-line setting after gemcitabine failure is scattered and scant [19]. The only established second-line regimen after failure of first-line gemcitabine in the metastatic setting is 5-fluorouracil with oxaliplatin based on the CONKO-003 trial. This phase III trial compared oxaliplatin and 5-fluorouracil with folinic acid vs. best supportive care as second-line therapy. The results showed a median overall survival of 40.0 weeks compared with 34.4 weeks after initiation of secondline chemotherapy (P=0.0312). It is notable that in this study, after 46 of 165 patients were randomized, the best supportive care arm was closed due to participating centers deciding that best supportive care alone was no longer acceptable [20]. This benefit, although statistically significant, is small and points to the dire need for more investigation.
At large this approach has not been successful and novel strategies are clearly needed. Concomitant administration of the monoclonal antibodies and tyrosine kinase inhibitors together and with combination chemotherapeutic agents may both augment their therapeutic activity as well as offset mechanisms of resistance.
We need to improve our knowledge on pancreatic cancer cells, relationships between tumoral, endothelial and stromal cells, and pancreatic cancer patients. Perhaps more importantly will be to truly target our therapy with the EGFR agents as well as other biologic agents by identifying those patients who are most likely to derive benefit and achieve meaningful responses. This is particularly crucial in a disease such as pancreatic cancer that has such a short life expectancy that the “window” for any given treatment may be quite small. Consequently, further study should include the development of more predictive assays and improved exploitation of surrogate biomarkers of response. We also need to need to study genomics and proteomics for individualized strategies. We definitely need to identify surrogates for survival. In addition the oncologists need to change their attitudes towards clinical trials (Figure 3) [21]. Development of novel agents and approaches are urgently needed in conjunction with improvement in access to clinical trials for patients.
The palliation of symptoms is arguably the most important goal in patients with locally advanced and metastatic disease. The distressing symptoms people with pancreatic cancer experience heighten the importance of early palliative-care intervention. At diagnosis patients often present with fatigue, loss of appetite, impaired sense of well-being, and pain. In addition to traditional palliative measures of managing pain and symptoms, surgery and endoscopy may in some instances play a role in palliation.
Pancreatic neuroendocrine tumors are a rare subgroup of tumors found in the pancreas which can be either functional or non-functional [22, 23, 24]. WHO classification classifies pNETs into:
• well differentiated tumors;
• well differentiated carcinomas; and
• poorly differentiated carcinomas;
in an attempt to predict natural history from the pathology report [22]. They are usually sporadic but they may also appear among other features of genetic syndromes like multiple endocrine neoplasia type I or von Hippel-Lindau disease.
Patients usually present with syndromes induced by hormones secreted from functional tumors, or with mass effects from non-functional tumors. Functional pNETs can secrete biologically active peptides like insulin, gastrin, glucagon, somatostatin, vasoactive intestinal polypeptide (VIP), whereas non-functional tumors also express and secrete peptides like neurotensin or chromogranin A, which are not active [22].
Most of the pNETs are already metastatic by the time they are diagnosed and liver is the most common site of metastasis. Regional lymph node spread is also common. PNETs are non-functional in their majority and the absence of a distinct functional syndrome, as well as their indolent course and subsequent delay in diagnosis, is mainly responsible for the advanced stage at the time of diagnosis [23, 24]. PNETs have a 5-year survival that can range from 97% in benign insulinomas to as low as 30% in non-functional metastatic pNETs [23, 24]. In addition, more recent data demonstrate that poorly differentiated pNETs can have similar prognosis with adenocarcinomas of the gastrointestinal tract [23].
Surgery with curative intent is the mainstay of treatment for localized or loco-regional disease [1, 2]. Surgery as well as other forms of local treatment like transarterial chemoembolization or radiofrequency ablation can also improve prognosis in patients with liver metastases [23, 25, 26]. For the inoperable cases, cytotoxic therapy with compounds like streptozotocin, 5-fluorouracil or doxorubicin can achieve modest outcome [27, 28, 29, 30, 31]. Treatment with somatostatin analogues like octreotide has been proven to prolong progression-free survival in patients with metastatic neuroendocrine tumors of midgut origin [32].
Two New Agents for the Treatment of pNETs
The U.S. Food and Drug Administration (FDA) approved two drugs Sutent® (sunitinib; Pfizer, New York, NY, USA) and Afinitor® (everolimus; Novartis Pharmaceuticals Co., East Hanover, NJ, USA) for the treatment of advanced pancreatic neuroendocrine tumors. It is exciting to see that the options available for patients with pNET are growing.
Sunitinib (previously known as SU11248) is an oral, small-molecule, multi-targeted receptor tyrosine kinase inhibitor. These include all receptors for plateletderived growth factor (PDGF-Rs) and vascular endothelial growth factor receptors (VEGFRs), which play a role in both tumor angiogenesis and tumor cell proliferation (Figure 4).
Therefore, the simultaneous inhibition of these targets leads to both reduced tumor vascularization and cancer cell death, and ultimately tumor shrinkage. FDA has approved Sutent® as the first anti-VEGFR therapy to treat progressive, well-differentiated pancreatic neuroendocrine tumors in patients with unresectable locally advanced or metastatic disease. This decision was based on the results of SUN 1111 pivotal phase III study. SUN 1111 is a randomized, double-blind, placebo-controlled phase 3 study (n=171) evaluating single-agent Sutent® in patients with unresectable pNET,. The primary endpoint was progression-free survival and secondary endpoints included overall survival, objective response rate and safety. Somatostatin analogs were permitted in the study.
The study demonstrated that Sutent® resulted in a significant improvement in progression-free survival compared to placebo (10.2 versus 5.4 months, P=0.000146) in this patient population [33]. Treatment with Sutent® also produced a statistically significant improvement in tumor response, with an objective response rate of 9.3% (95% confidence interval (CI): 3.2% to 15.4%; P=0.0066) versus no response with placebo. In addition, while overall survival was not mature at the time of final analysis, nine deaths were observed in patients enrolled in the Sutent® arm versus 21 deaths in patients enrolled in the placebo arm.
The most common adverse reactions were diarrhea, fatigue, asthenia, nausea, mucositis/stomatitis, anorexia, vomiting, neutropenia, hypertension, dyspepsia, abdominal pain, constipation, rash, handfoot syndrome, skin discoloration, hair color changes, altered taste and bleeding.
Sutent® is also approved for both gastrointestinal stromal tumors after disease progression on or intolerance to imatinib mesylate, and advanced renal cell carcinoma.
In addition, another targeted agent, Afinitor® (everolimus), mTOR inhibitor was approved for the treatment of patients with progressive pancreatic neuroendocrine tumors that are not resectable surgically, that are locally advanced or metastatic (Figure 5).
This approval by the FDA was based on a phase III clinical trial of Afinitor®, the RAD001 In Advanced Neuroendocrine Tumors (RADIANT)-3 trial. This study showed that treatment with Afinitor® resulted in median progression-free survival of 11.0 months versus 4.6 months with placebo and reduced the risk of cancer progression by 65% when compared with placebo in patients with advanced pancreatic pNET (hazard ratio=0.35; 95% CI: 0.27 to 0.45); P<0.001) [34]. Estimates of the proportion of patients who were alive and progression-free at 18 months were 34% (95% CI: 26% to 43%) with everolimus versus 9% (95% CI: 4% to 16%) with placebo. A consistent improvement in progression-free survival was seen with Afinitor® in all patient subgroups. The FDA determined that the safety and effectiveness of Afinitor® in the treatment of patients with carcinoid tumors have not been established.
The majority of drug-related adverse events (everolimus vs. placebo) were grade 1 or 2 and included stomatitis (64% vs. 17%), rash (49% vs. 10%), diarrhea (34% vs. 10%), fatigue (31% vs. 14%), and infections (23% vs. 6%). Grade 3 or 4 drug-related adverse events included: anemia (6% vs. 0%) and hyperglycemia (5% vs. 2%). Cases of hepatitis B reactivation and pulmonary embolism have been reported.
It is very exciting to see these new agents approved by FDA to treat patients with pNET. With this approval, U.S. physicians can now offer their patients with progressive pNET a new treatment helping to fulfill a critical unmet need.
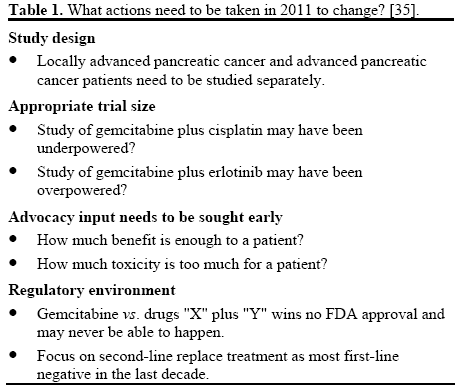
Pancreatic adenocarcinoma remains a treatmentrefractory cancer. Patients with any stage of pancreatic cancer can appropriately be considered candidates for clinical trials because of the poor response to chemotherapy, radiation therapy, and surgery as conventionally used. Given the limited treatment options, there is an urgent need for the development of novel agents that have the potential to impact survival rates and quality of life for the patients with special attention to neoadjuvant therapy, implementation of novel chemotherapy and radiation therapy studies (Table 1) [35].
Evaluation of targeted agents has been quite disappointing in the treatment of pancreatic cancer, except modest benefit of erlotinib. In addition to chemotherapy, the development of pancreatic cancer vaccines has been the subject of recent developments in the treatment of pancreatic cancer. Currently, two such vaccines are under clinical trials:
• algenpantucel-L immunotherapy to standard adjuvant therapy on survival in patients with resected pancreas cancer [36];
• GV1001 pancreatic cancer vaccine (TeloVac trial) [37].
Last but not the least, the late detection and poor prognosis of pancreatic cancer patients highlight the importance of an effective early detection strategy, especially for those at high risk of developing pancreatic cancer. Screening of high-risk patients with endoscopic ultrasound is gaining wider acceptance but evidence of efficacy and cost-effectiveness is still needed.
The author has no potential conflict of interest