- (2012) Volume 13, Issue 2
Muhammad Wasif Saif
Columbia University College of Physicians and Surgeons. New York, NY, USA
Adenocarcinoma; Biological Markers; Neoplastic Cells, Circulating; Pancreatic Neoplasms; Prognosis
Approximately 42,470 new cases of pancreatic cancer are diagnosed per year in USA, which represents approximately 3% of all newly diagnosed cancers [1]. Pancreatic cancer remains the fourth most common cause of cancer-related mortality in the United States. This close incidence to mortality ratio depicts the considerable diagnostic and therapeutic challenges faced by the patients as well as the care takers treating these patients. Sadly, more than 90% of patients diagnosed with pancreatic cancer succumb to their disease within 5 years; 75% within one year [2]. The major explanation for this poor prognosis is the lack of a therapeutic time window [3]. Pre- and early cancerous lesions are beyond our threshold of detection. Pancreatic cancer is diagnosed at an advanced disease in majority of the cases, and is further characterized with a high rate of local and distant recurrence following surgical resection, and relative chemo-resistance. Even an early stage tumor at the time of initial diagnosis can be metastatic and resistant to conventional therapies.
In the era of personalized medicine, understanding about premalignant lesions, knowledge of genetic abnormalities and development of targeted therapies we are often limited by the lack of tissue [4]. The tissue is extremely important to unveil the nature of the disease as well as to identify new molecular markers to predict outcome and response to treatment, and to aid in the detection of premalignant pancreatic lesions. This is of utmost importance as we have witnessed the integration of molecular markers and active therapies in other solid tumors, such as lung cancer. It is the prime time to do so for pancreatic adenocarcinoma.
The best chance of sampling tissue specimen is at the time of surgical resection. Unfortunately, only 10-20% patients are surgical candidates while the majority of patients with advanced disease are often diagnosed by fine-needle aspiration either via an EUS- or CT-guided biopsy, hence provide small sample. Due to this limitation only few cells are available which handicap any further investigation on the collected tissue.
Several cytotoxic and biological agents targeting epithelial tumor cells show promising results in preclinical and preliminary human studies but have failed to show relevant effects in larger randomized clinical studies. This discrepancy between experimental results and clinical results seem to be at least partly a result of the tumor microenvironment. Pancreatic ductal adenocarcinoma is characterized by remarkable desmoplasia, forming more than 80% of the tumor mass [5]. The desmoplasia is composed of extracellular matrix proteins, myofibroblastic pancreatic stellate cells, and immune cells associated with a multitude of cytokines, growth factors, and extracellular matrix metabolizing enzymes. We are just learning to appreciate the role of this complex process in carcinogenesis and resistance to chemotherapy. Investigators have shown that stellate cells produce extracellular matrix proteins, cytokines and growth factors that promote the growth of the cancer cells. Recent studies also suggest that interactions between extracellular matrix proteins and desmoplastic secreted growth factors with the cancer cells of pancreatic ductal adenocarcinoma activate intracellular signals including reactive oxygen species that act to make the cancer cells resistant to dying [6]. These findings suggest that the desmoplasia of pancreatic ductal adenocarcinoma is a key factor in regulating carcinogenesis of pancreatic ductal adenocarcinoma as well as responses to therapies.
It is clear that we are entering a new era of cancer therapy, in which molecular profiling of tumor specimens is likely to become routinely performed. This is made easier as the technology is more readily available. The incorporation of well-designed correlative studies into the design of therapeutic trials in pancreatic cancer therefore remains crucial to the advancement of this field. However, we have shown our failure to adequately collect tissue in major randomized phase III studies as well as cooperative group trials. Two such examples are:
• Radiation Therapy Oncol ogy Group (RTOG) 9704. Phase III randomized study of adjuvant fluorouracilbased chemoradiotherapy preceded and followed by fluorouracil versus gemcitabine in patients with resected adenocarcinoma of the pancreas: only 225 samples were found adequate from 538 patients after pancreatic resection [7].
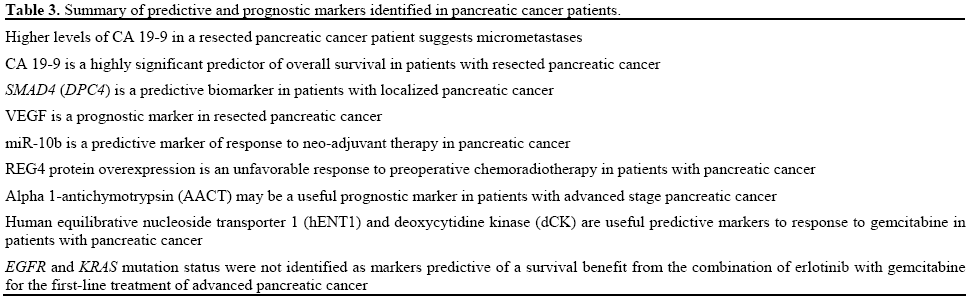
• National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG) PA.3. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: only 26% patients out of 569 had adequate tissue sampled [8]. Biomarkers are either prognostic or predictive (Table 1). Prognostic biomarkers are intrinsic indicators for tumor’s aggressiveness and patients’ final clinical outcome, regardless of the therapy received. Their clinical relevance is significant as they allow for better risk stratifications as well as rapid assessment of likelihood of disease progression or recurrence.

On the other hand, predictive markers are parameters used to predict treatment responses. Customized chemotherapies based on certain biomarkers have been shown to have better efficacy and result in improved outcome in cancer patients.
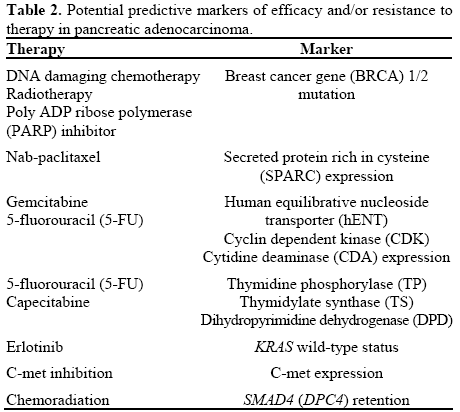
Several potential predictive biomarkers for cytotoxic therapy in pancreatic cancer have been identified, such as secreted protein rich in cysteine (SPARC) expression, KRAS, human equilibrative nucleoside transporter 1 (hENT-1), cytidine deaminase CDA), and cyclin-dependent kinase activity [11, 12, 13, 14, 15, 16, 17, 18] (Table 2). MicroRNA and cancer stem cells have also been identified as predictive biomarkers as well as potential therapeutic targets in this setting [19]. Predictive and prognostic markers identified in pancreatic cancer patients are summarized in Table 3.
Prospective collection of tissues mandated by the protocol in patients with pancreatic cancer is cumbersome and complicated for many reasons: fine needle aspiration offers a safe diagnostic test and provided ample tissue for confirmation of the diagnosis, but not enough to be further utilized for research purposes; core biopsies may add more risk to the patients; these procedures can add extra cost and if a repeat biopsy is needed then the cost can be doubled. Though these issues seem as a hindrance, we must overcome these barriers to move forward in the field of pancreatic cancer.
The ability to perform circulating tumor cells collection and proteomic biomarker profiling from serum samples may help us overcoming the traditional barriers to performing correlative studies. Circulating tumor cells are cancer cells that are detached from primary tumor sites and travel in the peripheral blood circulation system, leading to distant metastasis [20] (Figure 1). CTCs are typically enriched and detected via immunomagnetic separation system [21] or via microfluidic circulating tumor cell-chip system [22, 23].
De Albuquerque et al. [24] reported the prognostic values of CTCs detection in pancreatic adenocarcinoma. By using the high affinity antibodies BM7 (MUC 1) in addition to conventional VU1D9 (EpCAM), circulating tumor cell detection was reported in 49.3% of 144 peripheral blood samples from 39 patients with advanced pancreatic adenocarcinoma. The detection of such circulating tumor cells portended poor prognosis (median progression free survival: 60.7 days vs. 163.6 days in patients with positive and negative circulating tumor cell detections, respectively; P<0.0001). As such, authors concluded that circulating tumor cells can act as an independent prognostic biomarker.
The prognostic and predictive values of circulating tumor cells have been well established in breast and prostate cancer, though their utility in pancreatic cancer is very limited. As the technologies further advance, it is possible that circulating tumor cells may emerge as a critical prognostic as well as predictive biomarkers in pancreatic cancer [25].
Genome-wide analysis using high-throughput DNA method for potential molecular biomarker identifications and analysis is an attractive strategy in pharmacogenetics. Investigators have shown a promising set of single nucleotide polymorphisms, such as PYCARD and MACRE2, which appears to have strong positive correlation with efficacy from gemcitabine-based chemotherapy in pancreatic cancer [26].
Therefore, in view of data available, our clinical practice remains unchanged, though some of aforementioned biomarkers appear to have a potential prognostic and predictive role and have to be explored further. Given these promising preliminary data, future clinical trials using hybrid chemotherapy design [27], tailored towards standardized biomarker assay, may bring forward more insight and confirmatory data for this interesting concept.
Consensus report of the National Cancer Institute (NCI) Clinical Trials Planning Meeting on pancreas cancer treatment [28] pays emphasis on the enhancement of research to identify and validate the relevant targets and molecular pathways in pancreatic cancer, cancer stem cells, and the microenvironment. In addition, emphasis was also placed on developing rational combinations of targeted agents and the development of predictive biomarkers to assist selection of patient subsets. The report also recommends that phase III clinical trials should be implemented only if there is a meaningful clinical signal of efficacy and safety in the phase II setting. Therefore, the emphasis must be on performing welldesigned phase II studies with uniform sets of basic entry and evaluation criteria with survival as a primary endpoint. Patients with either metastatic or locally advanced pancreatic ductal adenocarcinoma must be studied separately.
A better understanding of the biology of desmoplasia in the mechanism of pancreatic ductal adenocarcinoma will likely provide significant opportunities for better treatments for this devastating cancer. Development of biorepositories in the conduct of randomized phase III trials in this disease is mandatory. This cannot be achieved without more funding and change in our approach and attitude towards research practice in the field of pancreatic cancer. Furthermore, improved awareness and understanding of hereditary genetic abnormalities predisposing to pancreatic adenocarcinoma present us the potential for both screening of at-risk individuals and development of molecularlytargeted treatment modalities. The pharmacogenomic studies have identified biomarkers of efficacy to established chemotherapy but prospective validation of these predictive and prognostic biomarkers need to be achieved immediately followed by their incorporation into clinical decision making.
The authors have no potential conflicts of interest