Review Article - (2018) Volume 0, Issue 0
1Chan Soon-Shiong Institute for Medicine, United States
2UC Irvine Medical Center, Division of Hematology Oncology, United States
Received Date: July 22nd, 2017; Accepted Date: September 24th, 2017
A physician should contemplate systemic therapy when patients are symptomatic, have significant tumor burden, or disease progression. Each patient and tumor is unique and there is no consensus on treatment sequencing and which regimen is the best. Over the past few years, a number of new agents are now approved for pancreatic neuroendocrine tumors and the treatment field has dramatically expanded. Our review will discuss the key agents and focus on the newer regimens. The treatment options range from sandostatin analogues, targeted treatments, and chemotherapy. We always recommend a multi-disciplinary team to help guide the treatments for pancreatic neuroendocrine tumors patients.
Everolimus; lanreotide; Octreotide; Streptozocin; sunitinib; temozolomide
PNETs pancreatic neuroendocrine tumors
Somatostatin Analogues
Somatostatin is a peptide hormone that regulates the endocrine system, affects cell proliferation, and inhibits the secretion of hormones in vivo. Somatostatin and its analogs will act by binding to somatostatin receptors, and inhibiting the secretion of peptides from NET cells through somatostatin receptor SSTR-2 and SSTR-5. The presence of these receptors is determined through diagnostic imaging with either an Octreoscan or Gallium- Ga DOTATATE. For those with positive imaging, symptoms can be well controlled with somatostatin analogs. They are highly effective in controlling symptoms in functional PNETs including VIPomas, glucagonomas, as well as somatostatinomas [1, 2, 3].
Current available Somatostatin analogues include Octreotide and Lanreotide. These analogues can mimic the physiological activities of somatostatin and can therefore inhibit secretion of hormones including gastrin, glucagon, insulin, TSH, VIP, thereby working to reduce secretion of fluids by the intestine and pancreas, reduce gastrointestinal motility, inhibit hormone action from the anterior pituitary and reduce portal pressure in bleeding varices [1, 2, 3]. A depot preparation known as Sandostatin LAR is considered the standard approach for symptomatic treatment. It is typically initiated a dose of 20 mg IM with dose-escalation. Patients may sometimes use short-acting octreotide for symptoms while doses are being titrated. Lanreotide is another synthetic analogue of somatostatin, binding to the same receptors as somatostatin, with a longer half-life and prolonged effects [3, 4, 5, 6, 7].
While Somatostatin analogs have a favorable safety profile, and are able to treat symptoms associated with hormone hypersecretion, the anti-proliferative effects to control tumor growth have continued to be examined. They are recommended in patients with unresectable, somatostatin receptor positive, well differentiated PNETs, with a high tumor burden [4, 5, 6, 7]. In the Controlled Study of Lanreotide Anti-proliferative Response in Neuroendocrine Tumors (CLARINET), the antiproliferative effects of Lanreotide were examined in 200 patients with nonfunctioning, somatostatin receptorpositive, unresectable locally advanced tumor or metastatic enteropancreatic neuroendocrine tumors, with Ki-67 values <10% [7, 8, 9, 10, 11]. In this 96-week, randomized, double-blind, placebo-controlled, multicenter, phase 3 study, an extended-release aqueous-gel formulation of Lanreotide at a dose of 120 mg, was compared with placebo through means of deep subcutaneous injection every 28 days. At 24 months, there was a significantly increased progression free survival (PFS) in the Lanreotide group at 65% (95% CI, 54.0 to 74.1) vs. 33% (95% CI, 23.0 to 43.3) in the placebo group. There were no significant differences in quality of life or overall survival [7, 8, 9, 10, 11].
In a separate trial from the PROMID Study Group, Octreotide LAR 30 mg intramuscularly was evaluated for tumor progression and survival in a placebo controlled, double blind, phase IIIB study performed in patients with well-differentiated metastatic midgut NETs. Results indicated that the median time to tumor progression, which was the primary efficacy end point in the octreotide LAR and placebo groups, was 14.3 and 6 months, respectively (hazard ratio [HR]=0.34; 95% CI, 0.20 to 0.59; P=0.000072). After 6 months of treatment, stable disease was observed in approximately 66% of patient in the octreotide group vs. 37% of patients in the placebo group. The HR for overall survival was 0.81 (95% CI, 0.30 to 2.18) [9, 10, 11, 12]. In order to then investigate whether this beneficial effect also affected overall survival patients in the PROMID trial, patients were then followed until January 2013 at least once a year. Between July 2001 and January 2008, 42 and 43 patients were randomly assigned to receive Octreotide or Placebo groups. Median OS for all 85 patients was 85 months, “not reached” in the Octreotide arm and 84 months in the Placebo arm (p=0.59, HR=0.85 [CI 0.46; 1.56. Median OS in the HL (hepatic tumor load) ≤ 10% subgroup was “not reached” (Octreotide) vs. 80.5 months (placebo) (p=0.14, HR=0.56 [CI 0.25; 1.23]). In the HL>10% subgroup the respective numbers were 35 vs. 84 months (p=0.14, HR=2.18 [CI 0.75; 6.33]). Conclusions from this follow up study indicated that Octreotide LAR not only prolonged Time to Progression, but also extended Overall Survival in the subgroup of patients with metastatic midgut and a low Hepatic Tumor Load (≤ 10% at study entry) but not in the high Hepatic Tumor Load (HL>10%) subgroup. Patients who had been randomized at study entry in the placebo group then received octreotide LAR after disease progression, but these experienced a less favorable OS in the low HL subgroup [10, 11, 12, 13, 14, 15].
Overall, somatostatin analogs have been effective in controlling symptoms associated with hormone hypersecretion in functional neuroendocrine tumors. In addition, they have also been shown to control tumor growth. Guidelines from the European Neuroendocrine Tumor Society and the North American Neuroendocrine Society suggest that in the asymptomatic setting these medications should be initiated for unresectable, somatostatin receptor positive, well differentiated PNETs with high tumor burden. Although progression free survival has been correlated with treatment, overall survival with somatostatin analogs continues to be under clinical investigation [12, 13, 14, 15, 16, 17, 18].
Small Molecule Tyrosine Kinase Inhibitors (TKI)
Sunitinib: Vascular endothelial growth factor (VEGF) is a crucial driver of angiogenesis in PNETs [19, 20]. Tissue from malignant PNETs has widespread expression of platelet-derived growth factor receptors (PDGFRs) α and β, stem-cell factor receptor (c-kit), and vascular endothelial growth factor (VEGF) receptors VEGFR-2 and VEGFR-3 [21, 22, 23]. Preclinical studies have shown that Sunitinib malate (Sutent, Pfizer) inhibits the aforementioned kinases [24, 25], and delays tumor growth of pancreatic islet-cell tumors in transgenic mouse models [26, 27]. In phase 1 and 2 trials, Sunitinib showed antitumor activity in patients with PNETs [26, 27]. In a phase III trial in patients with advanced, well-differentiated, progressive PNETs, a total of 171 patients were randomly assigned (in a 1:1 ratio) to receive best supportive care (BSC) with either Sunitinib at a dose of 37.5 mg per day or placebo [28]. The study was discontinued early because the independent data and safety monitoring committee observed more serious adverse events and deaths in the placebo group as well as a difference in progression-free survival (PFS) favoring Sunitinib. Sunitinib improved investigatorassessed PFS versus placebo (11.4 vs. 5.5 months; HR, 0.42; P<0.001). Median OS was not reached, however the HR for death was 0.41 (95% CI, 0.19 to 0.89; P=0.02) in favor of Sunitinib. Hand-foot skin reaction and hypertension of any grade occurred in 23 and 26% of patients receiving Sunitinib, respectively, and the most common grade 3 or 4 adverse events in this group were neutropenia (12%) and hypertension (10%). Despite these side effects, there were no differences in the quality-of-life index with Sunitinib. Based upon these data, Sunitinib was approved by the U.S. Food and Drug Administration (FDA) in May 2011 for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumors in patients with unresectable, locally advanced, or metastatic disease. In a later report, 5 years after study closure median OS was 38.6 months in the Sunitinib group versus 29.1 months for placebo (HR, 0.73; 95% CI 0.50-1.06; P=0.094). Although the observed median OS improved by nearly 10 months, it did not reach statistical significance, potentially due to crossover from placebo to Sunitinib in 69% of the patients in the control group [29].
Mammalian Target of Rapamycin (mTOR) Inhibitors
Everolimus: Everolimus (Afinitor, Novartis Pharmaceuticals) inhibits mammalian target of rapamycin (mTOR), a serine–threonine kinase which stimulates cell growth, proliferation, and angiogenesis [30, 31, 32]. Autocrine activation of the mTOR signaling pathway, mediated through insulin-like growth factor 1, has been implicated in the proliferation of PNETs [33]. Inhibition of mTOR has anti-proliferative effect on PNET cell lines [34, 35]. Everolimus has demonstrated promising antitumor activity in phase 2 studies involving patients with PNETs [30, 31].
The RAD001 in Advanced Neuroendocrine Tumors, third trial (RADIANT-3) study was an international, multicenter, double-blind, phase 3 study that compared Everolimus monotherapy (10 mg daily) to placebo both in conjunction with BSC in 410 patients with advanced PNETs [36]. The median PFS was 11.0 months with Everolimus as compared with 4.6 months with placebo (HR for disease progression or death from any cause with Everolimus, 0.35; 95% confidence interval [CI], 0.27 to 0.45; P<0.001), representing a 65% reduction in the estimated risk of progression or death. Median OS was not reached at the time of study reporting, and no significant difference between the groups was observed (HR for death with Everolimus, 1.05; 95% CI, 0.71 to 1.55; P=0.59). The most common grade 3 or 4 drug-related adverse events were stomatitis (7%), anemia (6%), and hyperglycemia (5%). Based upon these data Everolimus was approved in the United States for the treatment of progressive NETs of pancreatic origin in patients with unresectable, locally advanced, or metastatic disease in May 2011. In a later analysis, median OS favored Everolimus (44 versus 37.7 months), but the difference was not statistically significant (hazard ratio, 0.94; 95% CI, 0.73 to 1.20; P=0.30), likely secondary to the high rate of crossover of patients from placebo to Everolimus (85 percent) which may have confounded the ability to detect a difference in OS [37].
Somatostatin analogs, Everolimus, and Sunitinib all extend PFS compared with BSC alone, although none of these agents have been compared directly with each other. In the absence of comparative trials, the choice of initial agent is influenced by the adverse effect profile. Due to a favorable toxicity profile; somatostatin analogue may be an appropriate first choice for many patients with use of targeted agents such as Sunitinib or Everolimus upon disease progression [38, 39]. Since Everolimus causes hyperglycemia, it has an additional therapeutic benefit in patients with functioning insulinomas and refractory hypoglycemia [40, 41].
Streptozocin (STZ)
Although the results of chemotherapy in extrapancreatic NETs are often disappointing resulting in objective response rates (ORR) of less than 20% in most trials, PNETs are known to be chemosensitive [39, 42]. Streptozocin (STZ) based combination therapy has been a historical treatment standard for patients with advanced PNETs [42]. In retrospective studies, STZ-based chemotherapy regimens, in which STZ is combined with doxorubicin, fluorouracil, or both, have been associated with ORR of 30% to 40% [43, 44]. However, widespread use of STZ which is an alkylating agent has been limited by the cumbersome administration schedule and by concerns about toxicity [42]. In addition, with the availability of targeted agents, the role of systemic chemotherapy for PNETs remains controversial and the sequence of treatment remains unclear [44].
Temozolomide
Recently, several retrospective series and small prospective studies have explored another alkylating agent Temozolomide –based regimens in patients with advanced PNETs, and demonstrated ORR to be comparable to those observed with STZ-based therapy [39]. In a small retrospective study of 30 patients, the combination of Temozolomide with Capecitabine (CAPTEM) showed an impressive ORR of 70% [45]. More recently several small retrospective studies with CAPTEM demonstrate an ORR of 43%–70%, corresponding to a clinical benefit of 12–18 months in terms of PFS and a very impressive disease control rate (DCR) of 70%–97% [46, 47, 48, 49, 50, 51, 52]. A preliminary report of a prospective phase II trial reported at the 2014 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium noted an objective partial response in 4 of 11 patients with advanced PNETs (36%) and an ongoing PFS in PNETs of >18.2 months which is 150% greater than reported with Everolimus and Sutent [48]. A trial conducted by Eastern Oncology Cooperative Group (ECOG) which has completed enrollment, is evaluating the relative efficacy of the Temozolomide plus Capecitabine versus Temozolomide alone (ClinicalTrials. gov Identifier: NCT01824875). Why some PNETs and not others are susceptible to treatment with alkylating agents remains uncertain. Several small retrospective series have suggested that O6-methylguanine DNA methyltransferase (MGMT) deficiency may predict response to Temozolomide in PNETs as well; however expression of MGMT has not been validated as a predictive biomarker [39, 53]. The question whether MGMT status could be a response predictor to CAPTEM therapy was updated at the ASCO meeting in 2015. In a trial with 144 PNETs, MGMT status was not predictive of response (P=0.358) [54]. Conversely, MGMT methylation was correlated with PFS prolongation (16.3 vs. 5.4 months) in PNET patients in another study presented at the same meeting [53]. Due to lack of prospective validation as a predictor of response in NETs, and variability in the techniques used to assess MGMT status, it cannot be put to routine clinical use to select patients for Temozolomide therapy.
Dacarbazine
Dacarbazine, an alkylating agent, has also been used to treat PNETs. It has been assessed alone and in combination with 5-FU, epirubicin, leucovorin, and other agents. A phase II study of Dacarbazine alone was conducted in 50 PNET patients with a response rate of 34% and a survival time of 19.3 months. However, not unlike STZ, the toxicity of Dacarbazine has limited its widespread use [55, 56].
Other Drugs
In addition to the alkylating agents described above, other antineoplastic drugs, including 5-FU, paclitaxel, gemcitabine, and oxaliplatin, have been used in clinical studies to treat PNETs. However these studies were not randomized and were conducted using small numbers of patients. Some studies have described the use of oxaliplatin in combination with capecitabine in PNETs, with response rates of about 30%, a median PFS of 9.8 months, and a median survival time of more than 24 months [56].
Sequence of Therapies and Future Direction
As discussed in other sections of this review, for patients with potentially resectable metastatic disease, resection may provide control of symptoms and prolong survival. For patients with unresectable disease, options to control tumor growth and symptoms related to hormonal hypersecretion include somatostatin analogues, nonsurgical liver-directed therapy, and systemic antitumor therapy [38]. Some patients with PNETs may feel relatively well despite the presence of metastatic disease. Such patients with asymptomatic advanced pancreatic NETs, who otherwise have low-grade tumor histology and a minimal volume of metastatic disease, can likely be managed by diligent observation alone rather than committing them to a lifetime of antineoplastic therapy. Patients with symptoms of hormone hypersecretion should be managed with somatostatin analogs and other agents, as appropriate to the specific syndrome as detailed in other sections of this review [38, 39]. Moreover, somatostatin analogs also have an anti-proliferative tumor effect in functional and non-functional PNETs [8].
For patients with a larger disease volume or evidence of tumor progression, treatment should be initiated even if the patient is asymptomatic. Somatostatin analogs, Everolimus, and Sunitinib all extend PFS compared with BSC alone, although none of these agents have been compared directly with each other. In the absence of comparative trials, the choice of initial agent is influenced by the adverse effect profile. Due to a favorable toxicity profile; somatostatin analog may be an appropriate first choice for many patients with use of targeted agents such as Sunitinib or Everolimus upon disease progression [38, 39].
With the proven benefit of molecularly targeted drugs, including Everolimus and Sunitinib in large-scale randomized controlled studies, these agents have been accepted as a standard therapy, and the place of cytotoxic chemotherapy in the treatment of PNETs has become even more uncertain. As such cytotoxic chemotherapy should be used for patients who cannot tolerate Everolimus or Sunitinib or have failed to respond to these drugs. Due to lack of data from large prospective randomized trials, no cytotoxic chemotherapy regimen has been established as a global standard [38, 39, 56]. Most frequently employed regimens include alkylating agents, such as Streptozocin and Temozolomide, of which Temozolomide based regimens (for example CAPTEM) appear to have a better side effect profile [53].
The response rates to molecularly targeted drugs are relatively low (Sunitinib, 9.3%; Everolimus, 5%), and a cytoreductive effect cannot be expected. Conversely, cytotoxic chemotherapy is associated with higher tumor response rates than either somatostatin analogues or the targeted therapies. As a result, for patients who are highly symptomatic from tumor bulk or who have rapidly enlarging metastases, and for whom tumor shrinkage rather than tumor stabilization is the primary objective, front line cytotoxic chemotherapy is the preferred option rather than molecularly targeted therapy or a somatostatin analog [38, 39, 56, 57].
Several critical questions remain unanswered and need to be elucidated via large scale multi center randomized studies given the rarity of this disease. Sunitinib, Everolimus and somatostatin analogues should be compared directly to each other in a prospective trial. At the present time, the evidence for cytotoxic chemotherapy as a standard therapy is inadequate, but there is a strong possibility that its usefulness will be demonstrated, and diligent evaluation using high-quality clinical trials is needed. Although there is no head-to-head comparison between CAPTEM and molecularly targeted drugs in PNETs, and cross trial comparison is not prudent, the median PFS and ORR achieved by CAPTEM (12–18 months, 43-70%) is superior to that produced by targeted drugs (11 months, <10%). Therefore CAPTEM should be tested against Sunitinib and Everolimus in the first-line setting [53]. Trials on the best sequence of treatments, e.g. the SEQTOR trial, a European randomized phase III study investigating STZ+5-FU followed by Everolimus versus the reverse sequence, are ongoing (ClinicalTrials.gov Identifier: NCT02246127) (Table 1).
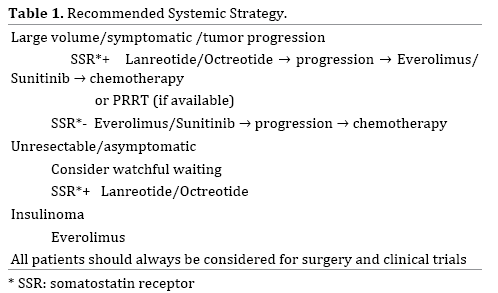
Therapeutic resistance involving a multitude of mechanisms is often encountered with targeted agents. Concurrent inhibition of VEGF and the mTOR pathway may be an effective strategy to overcome resistance [38]. In a Phase II study, combination therapy with mTOR inhibitor temsirolimus and VEGF-A antibody bevacizumab showed an RR of 41% [58]. Another phase II study that randomized patients with metastatic PNETs to receive everolimus with or without bevacizumab showed a higher RR in the combination group (31% vs. 12%, P=0.005) [58]. However, severe adverse events were more frequent in the combination group, highlighting the need for less toxic regimens. The antiangiogenic TKI pazopanib demonstrated promising results in a phase II trial where PNET patients who received pazopanib combined with depot octreotide had a median PFS of 14.4 months [59].
Although these new agents give patients multiple therapeutic options, treatment stratification becomes critical. To date, no established predictive markers are available to facilitate treatment decisions and to identify the optimum agent for each patient. Clarifying which patients should be treated and the optimal timing of the treatment should also be addressed in the future trials. The proliferation marker Ki-67 is well known as a prognostic marker; however its role as a predictor of response to treatment is less well defined [42]. In a study by Turner et al. both the mitotic index and Ki67 were associated with a response to chemotherapy consisting of 5-fluorouracil, cisplatin, and streptozocin [60]. In another study of PNET patients, 28 tumor with ki67>5% showed an ORR of 64% versus 29% for the 31 PNETs with ki67<5%, P=0.006 [53,54]. Similarly, another trial of NETs described an ORR of 29% in tumors with ki-67<2% and 39% in NETs with ki- 67>2% to 20% [52]. However definite conclusions cannot be made from these small retrospective studies.
Peptide Receptor Radionuclide Therapy (PRRT)
PRRT is a form of systemic radiotherapy that allows targeted delivery of radionuclides to tumor cells expressing high levels of somatostatin receptors. Historically, the first therapeutic experiences in NET were performed using 111ln-pentetreotide and produced a clinical benefit but rarely a radiological response [61]. The two radiopeptides most commonly used for PRRT, 90Y-DOTA0-Tyr3-octreotide (90Y-DOTATOC) and 177Lu-DOTA0-Tyr3-octreotide (177Lu- DOTATATE), have been successfully employed for more than a decade for the treatment of advanced NET. They differ from one another in terms of emitted particles, particle energy, and tissue penetration [62, 63, 64].
90Y is a high-energy β-particle emitter. The most extensive experience with 90Y-DOTATOC comes from a large single institution series of 1109 patients with metastatic gastroenteropancreatic NET and disease progression within 12 months of study entry, with visible tumor uptake on pretreatment somatostatin receptor scintigraphy [65]. The median number of courses administered was two, range 1 to 10. Overall, 378 patients (34%) had a "morphologic" response, 172 (15%) had a biochemical response, and 329 (29.7%) improved symptomatically. The median survival from diagnosis was 94.6 months. Longer survival correlated with responses by any of the above criteria. Transient grade 3 or 4 hematologic toxicities developed in 142 (12%), and loss of renal function was the main dose limiting toxicity. In all, 103 patients (9%) had permanent grade 4 or 5 (fatal, n=35) renal toxicity. 177-Lu emits both β and γ rays. Data from non-randomized trials of 177-Lu-Dotatate have consistently shown high response rates and long durations of median progressionfree survival in heterogeneous patient populations with gastroenteropancreatic neuroendocrine tumors [66, 67, 68, 69]. Response rates were particularly high in PNETs, ranging from 36% for nonfunctioning tumors, to approximately 40 to 60% for functioning gastrinomas, insulinomas, and VIPomas.
A high prevalence of somatostatin receptor expression in NETs provides the rationale for PRRT. The role of PRRT in patients with progressive advanced PNETs is unclear. In the United States, radiolabeled somatostatin analogs are not widely available, and this form of treatment for PNETs remains investigational. Here are reports of several PRRT studies in PNETs.
A prospective phase II study published in 2013 used 177Lu-octreotateina cohort (n=52) with advanced well or moderately differentiated PNETs. Patients were divided into two groups treated with different levels of activity based on possible existence of risk factors for renal toxicity, such as hypertension and diabetes. Thus, full dose (21-28 GBq) was compared with a reduced dose (11-20 GBq) for a normal and risk subset of subjects, respectively. Both regimens resulted in antitumor efficacy. PFS was not reached at the time of the analysis in the cohort treated with the full-dose regimen, whereas it was 20 months in individuals treated with a reduced dose. This suggests the full-dose scheme should be recommended, whenever possible [70]. After three more years follow up of the same study, 60 consecutive patients with PNETs were enrolled. Eligible patients were treated with two different total cumulative activities (18.5 or 27.8 GBq in 5 cycles every 6–8 weeks), according to kidney and bone marrow parameters. 28 patients received a mean full activity (FA) of 25.9 GBq and 32 a mean reduced activity (RA) of 18.5 GBq. The DCR, defined as the sum of CR+PR+SD was 85.7% in the FA group and 78.1% in the RA group. Median PFS was 53.4 months in the FA group and 21.7 months in the RA group (P=0.353). Median OS was not reached in FA patients and was 63.8 months in the RA group (P=0.007). Furthermore, 55 patients underwent an FDG PET scan before Lu-PRRT, 32 (58%) showing an increased FDG uptake in tumor sites. mPFS was 21.1 months in FDG PET positive patients and 68.7 months in the FDG PET-negative group (P<0.0002), regardless of the total activity administered. Although FA and RA are active in patients undergoing Lu-PRRT, a FA of 27.8 GBq of Lu-PRRT prolongs PFS and OS compared to an RA of 18.5 GBq. Also it indicated that FDG PET is an independent prognostic factor [71].
A retrospective study evaluated a cohort with metastatic PNETs (n=68, 52% at their first systemic treatment) treated with 177Lu-octreotate (four intended cycles, 8 GBq each, at 3-month intervals). Partial responses were noted in 60%, with a median PFS of 34 months. Multivariate analysis indicated that G1 tumors had a longer PFS [68]. Even individuals with Ki67>10% benefited from PRRT, with a median PFS of 19 months as opposed to 26 months for the entire cohort [72].
Another separate study by van Vliet et al. included 29 non-resectable or borderline resectable or oligometastatic (≤3 liver metastases) nonfunctioning PNETs. This group was treated with 177Lu-octreotate with neoadjuvant intent. After PRRT, successful surgery could be performed in 9 patients (31%). PFS was significantly longer in operated patients (69 vs. 49 months). A further comparison with 90 pluri-metastatic subjects treated in the same fashion provided a PFS of 25 months [73]. This study supports the proposal of early treatment and the possibility of down staging tumors with PRRT.
Where available, PRRT could be considered for patients with tumors that express somatostatin receptors and are otherwise refractory to medical therapy. Randomized, prospective studies to better define anti-tumor activity and long-term toxicity of radiolabeled somatostatin analogs in PNETs are needed.
We declare that we have no conflict of interests.