Case Report - (2016) Volume 17, Issue 3
Nader El-Sourani, Helge Bruns, Dalibor Antolovic, Hans-Rudolf Raab
University Hospital for Visceral – and General Surgery, Klinikum Oldenburg GmbH, Rahel-Straus-Str.10, 26133 Oldenburg, Germany
*Corresponding Author:
Nader El-Sourani
University Hospital for Visceral and General Surgery
Klinikum Oldenburg GmbH
Rahel-Straus-Str.10, 26133 Oldenburg
Germany
Phone +0049-441-403-77628
E-mail el-sourani.nader@klinikum-oldenburg.de
Received September 30th, 2015-Accepted December 30th, 2015
Schwannoma is a well-defined benign tumor which arises from neural crest cells and surround the nerve sheath. Schwannoma of the pancreas is extremely rare, and only a limited number of cases have been reported in the literature as of today. Schwannomas of the pancreatic tail are extremely rare (4%). We hereby report a case of a patient undergoing distal pancreatectomy with en-bloc splenectomy for a cystic lesion of the pancreatic tail. The diagnosis of a pancreatic schwannoma was made upon histological examination.
Keywords
Neurilemmoma; Pancreas; Pancreatectomy
Abbrevations
SPT solid pseudopapillary tumors
INTRODUCTION
Pancreatic schwannomas are rare neoplasms that originate from Schwann cells. Usually, schwannomas occur in the peripheral nerve sheath of the extremities. However, visceral localization of these neoplasms, specifically pancreatic schwannomas arise from either parasympathetic or sympathetic fibres from branches of the vagus nerve coursing through the pancreas [1]. Pancreatic schwannomas affect adults with an equal gender distribution and vary considerably in size. Approximately two thirds are reported to undergo degenerative changes including calcification, haemorrhage, hyalinization, cyst formation and xanthomatous infiltration. Due to its potential of cystic degeneration, pancreatic schwannomas can radiologically mimic other cystic pancreatic lesions. This report presents a patient with a tumor of the pancreatic tail presenting with abdominal pain. The tumor was diagnosed as a schwannoma.
CASE REPORT
A sixty-nine-year-old female patient presented with diffuse abdominal pain to her local GP-clinic. The patient denied symptoms such as fever, weight loss or night sweats. The past surgical history revealed an open cholecystectomy 20 years ago. During the abdominal ultrasound an epigastric mass was detected and the patient was referred to our university hospital for further investigation
Upon clinical examination the abdomen was soft without any pain or tenderness. No abdominal masses were palpable. An incisional hernia was diagnosed after open cholecystectomy about 20 years ago. Further physical examination was normal. All laboratory findings except for a CEA of 3.4 μg/L were normal. Tumor marker CA19-9 was normal.
Due to an unknown mass revealed on ultrasound a computed tomography (CT) of the abdomen and pelvis was performed which described a 2.2 cm large well-encapsulated tumor with cystic areas in the tail of the pancreas (Figure 1). Neither liver metastases nor peri-pancreatic lymph node swellings were detected. Endoscopic ultrasonography showed a 2.8×2.5 cm large hypoechoic, partly inhomogeneous well-encapsulated and highly vascularised tumor composed of solid and cystic parts.
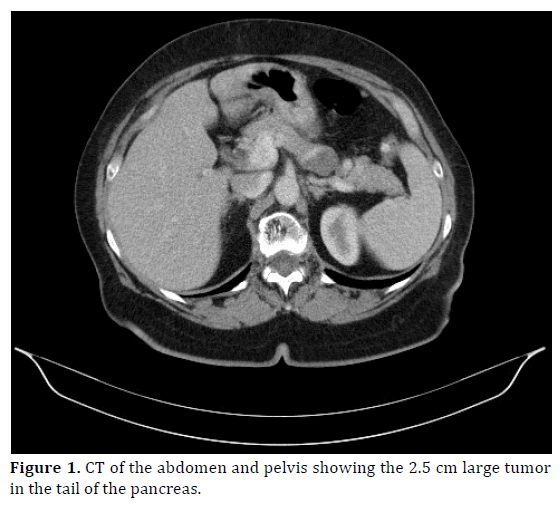
Figure 1. CT of the abdomen and pelvis showing the 2.5 cm large tumor in the tail of the pancreas.
Due to an unknown tumor mass (DD: neuroendocrine tumor) in the pancreatic tail it was indicated to perform surgery. Intraoperative a distal pancreatectomy with en-bloc splenectomy was carried out. On macroscopic examination the tumor was 2.5 cm large with cystic areas in close proximity to the splenic artery. On microscopic examination the tumor was composed of spindle shaped cells with wavy nuclei in palisading and interlacing fashion, strongly positive for S-100 proteins. The safety margins were tumor free. All peripancreatic lymph nodes were tumor free. The tumor cells were negative for smooth muscle actin. Therefore, the tumor was histologically diagnosed as a benign schwannoma of the pancreas. The patient was discharged uneventfully on the 7th postoperative day.
DISCUSSION
A schwannoma was first described by Verocay in 1910 who reported a schwannoma as a true neoplasm that originated from the Schwann cells, and which did not contain neuroganglion cells [2]. The anatomic distribution of schwannomas varies widely, however, schwannomas usually occur in the extremities. Other sites include trunk, head and neck, retroperitoneum, mediastinum, pelvis and rectum [3-5]. Pancreatic schwannomas are very rare neoplasm that arise from either parasympathetic or autonomic sympathetic fibres, both of which course through the pancreas as branches of the vagus nerve [6-10]. The majority of pancreatic schwannomas are located in the head (40%) and body (21%) of the pancreas. Only 4% of pancreatic schwannomas are located in the tail of the pancreas [11].
Microscopically schwannomas are encapsulated neoplasms that are composed of hypercellular (Antoni A) and hypocellular (Antoni B) areas. Antoni A areas are characterized by closely packed spindle cells with nuclear palisading and Verocay bodies, whereas the Antoni B areas are occupied by loosely arranged tumor cells. Degenerative changes like hyalinisation, cystic changes, haemorrhage or xanthomatous calcification are often recognized in the Antoni B areas [12, 13]. These degenerative changes are often due to necrosis and vascular thrombosis. Immunohistochemically, pancreatic schwannomas are positive for S-100 protein, CD 56 and vimentin [14]. Cystic pancreatic schwannomas can mimic the whole spectrum of pancreatic lesions such as: intraductal mucinouspapillary neoplasms, serous cystic neoplasms, mucinous cystic neoplasms, solid and pseudo-papillary neoplasms, lymphangiomas and pancreatic pseudocysts.
The most common symptom was described to be abdominal pain (57%). 30% of diagnosed patients were asymptomatic and the lesions were incidentally discovered on CT scans performed for other reasons. Other symptoms include weight loss (13%), back pain (6%), nausea/ vomiting (4%), melena (4%) and jaundice (4%) [15, 16].
Preoperative diagnosis of pancreatic schwannomas is very difficult even with recent advances in radiological imaging. Suzuki et al. reviewed the literature with special references to imaging features identifying an area of low density and/or cystic images reflecting the Antoni B component or degenerative cystic areas of the schwannoma as the most characteristic feature on a CT scan [17]. MRI findings usually show hypointensity on T1- images and hyperintensitiy on T2-images. However, all of these radiological features are not specific for pancreatic schwannomas as they often share those imaging features with other pancreatic tumors [18, 19]. Ultrasound-guided fine needle aspiration (EUS-FNA) has been increasingly used as a preoperative diagnostic tool, however, the effectiveness is questionable due to insufficient specimen collection and defection in collection technique.
Since schwannoma is a benign entity and malignant transformation is uncommon, enucleation of the tumor is usually sufficient [11]. Establishing the diagnosis prior to surgery is imperative because the treatment of a benign schwannoma is enucleation as compared to radical surgery. However, as preoperative diagnosis is very difficult most common resections include: pancreaticoduodenectomy (32%), distal pancreatectomy (21%) and enucleation (15%) [11].
In conclusion, pancreatic schwannoma is a rare but relevant entity with regard to the differential diagnosis of pancreatic lesions. Preoperative diagnosis is crucial, however it is very difficult. For benign lesions, simple enucleation of the tumor is adequate, whereas malignant tumors require standard oncological resection.
Conflict of Interest
The authors declare that they have no competing interests.