Research Article - (2024) Volume 9, Issue 2
Received: 09-Jun-2020, Manuscript No. IPPS-24-4603; Editor assigned: 12-Jun-2020, Pre QC No. IPPS-24-4603 (PQ); Reviewed: 26-Jun-2020, QC No. IPPS-24-4603; Revised: 01-Jul-2024, Manuscript No. IPPS-24-4603 (R); Published: 29-Jul-2024, DOI: 10.35841/2471-9935-9.2.11
The present study has been achieved in three stages. The first stage involves the preparation of three acrylate monomer, the first one is 2, 2-dimethyl-1,3-dioxoylan-4-yl-methylacrylate (Solketal Acrylate) (SKA) and was prepared in two steps. The other two monomers act as functional and hydrophobic, 4- Acetylphenyl Acrylate (APA) and 4-formyl-2-methoxyphenylacrylate (VA) which prepared from vanillin; both prepared in a one-step reaction. All monomers were evaluated by 1H, 13C NMR and FTIR. In the next stage, two kinds of polymers have been fabricated. Free radical copolymerization of SKA with a different molar concentration of photo-cross-linker producing poly (SKA-co-DMIAAm), while the copolymerization between N-isopropylacrylamide with a different molar concentration of VA and APA for producing the thermo-responsive functional polymer. All polymers have been characterized chemically and physically. The lower critical solution temperature of thermo-responsive polymers was measured. Finally, the formation of gel thin films by deposition of photo-cross-linked polymer over gold thin film then cross-linked by UV irradiation was developed. The surface was chemically modified by the creation of hydroxyl groups. An additional layer of thermo-responsive functional polymer thin film was performed. The swelling of each layer was measured using surface plasmon resonance with an optical waveguide. The aim of the study is the formation of a gel multi-layer with a hydrophobic and hydrophilic character with a thermo-responsive upper layer and a non-responsive lower layer that facilitate the formation of gel vessel for targeting biomolecules.
Environmental polymer and hydrogel; Photo-cross-linked solketal acrylate; Gel thin films; Surface plasmon resonance
In recent years materials with unique properties that can change their properties with the response to the surrounding environment have been great interests, as these polymers and hydrogels, materials were called environmental, responsive and stimuli-responsive and smart. There are several kinds of responsiveness e.g. temperature, pH, light, ionic strength and shape. A dual responsive has been prepared by a combination of two or more monomers or polymer with different stimuliresponsiveness. N-Isopropylacrylamide (NIPAAm) has considered as the most familiar thermo-responsive monomer and was used for the preparation of many thermo-responsive polymers and hydrogels; it has a Lower Critical Solution Temperature (LCST) or phase separation at 32℃ of that is closed to the temperature of the human body. 3D polymer network is called gel or hydrogel (aqua-gel) in the case of hydrophilic polymers that are capable to absorb a large amount of water. The importance of gel technology has been widely spread attributed to highly successful applications in the industrial field e.g. cell culture, self-healing and drug delivery [1].
Thermo-responsive hydrogel based on Nisopropylacrylamide has been attended by many scientists. Several articles have been focused on the preparation of thermo-responsive micro/nanogels and studied the swelling properties in water by heating and cooling as a reversible process. The important of glycerol in the industrial field has been increased. It can be modified to several organic compounds; one of the most important modifications is the formation of solketal. Solketal monomer is one of the keys to getting polymers and hydrogel with a reactive hydroxyl group. The hydrolysis of solketal acrylate was used to produce glycerol monomethacrylate or 2, 3-dihydroxypropyl methacrylate which is highly hydrophilic monomer and base a constituent in the biocompatible gel network. The presences of hydroxyl groups in glycerol monomethacrylate encourage the material scientist to use it instead of 2-Hydroxyethyl Methacrylate (HEMA) in the fabrication of contact lenses and photoresists. In the last decades, many reactions and polymerization have been improved to solketal acrylate e.g. Atom Transfer Radical Polymerization (ATRP) and grafting. The introduction of hydrophobic monomer into the thermoresponsive polymers or gel will affect the lower critical solution temperature towards lower values.
Vanillin has a great interest in the preparation of functional monomer due to the reactivity of both aldehyde and hydroxyl groups. Their derivatives can be easily separated and collected. A recent article has been prepared vanillin derivative and used for the synthesis of the bio-based polymer. The functional gel is a kind of gel that contains at least one functional group and has the ability to make chemical modifications; it has been utilized in several routes like click chemistry, ester chemistry, polymeric anhydrides, epoxides, aldehydes and ketones. Michael type and Friedel-crafts reactions, as well as methylations, have been used. The reactivity of the functional group was used in the formation of binding with a variety of biomolecules that is applicable in the biosensor and chromatographic methods. Moreover, the advanced medication used the attachment and release of biomolecules in polymer-drug delivery. The formation of hydrogel thin film with the functional group and stimuli-responsive layer has been recently studied; the swelling properties were measured by using Surface Plasmon Resonance spectroscopy with Optical Waveguide spectroscopy (SPR/OW). The technology of (SPR/OW) was used to investigate the phase separation and the lower critical solution temperature of the swollen hydrogel in water and different pH as a function of refractive index or volume degree of swelling with temperature variation. Many articles were published and discussed the applications of the responsive hydrogel in biological and medical technology e.g. the sensor (biosensor), biotechnology and switchable wettability.
In this work, we focused on the fabrication of functional hydrogel bilayer by assembly layer-by-layer with hydrophilic/hydrophobic chains, this kind of hydrogel can be used as a biological vessel to attach and release biomolecules.
Glycerol (99%, Merck, Germany), 4-hydroxyacetophenone (98%, Aldrich, Germany) 2, 2’-Azobis (Isobutyronitrile) (AIBN) (96%, Acros, Germany) Acryloyl chloride (99%, Merck, Germany), di-tert-butyldicarbonate ((boc)2O) (98%, Acros, Germany) diaminoethane (97%, Fluka, Germany), dimethyl maleic anhydride (98%, Aldrich, Germany), NIsopropylacrylamide (NIPAAm) (97%, Fluke, Germany) was recrystallized from distilled n-hexane. Dimethyl maleic anhydride (98%, Aldrich, Germany), was recrystallized from methanol. Vanillin (99%, Aldrich, Germany), di-tertbutylamine (97%, Aldrich, Germany), thioxanthone (Acros), triethylamine (Merck), sodium carbonate (Grussing), magnesium sulphate (Merck). Dioxane, Tetrahydrofuran (THF), Dichloromethane (DCM) and diethylether were distilled over potassium hydroxide, gold (Au, 99.999%, Aldrich Germany), cyclohexanone (95%, Fluke, Germany) was purified by distillation after drying overnight in magnesium sulphate. Other chemicals were used as received.
Instrumentations
The 1H NMR and 13C NMR spectra were recorded on a Bruker AVANCE 500 spectrometer (500 MHz). IR spectra were recorded on VERTEX 70 Fourier transform infrared instrument. The samples were milled with KBr pellet. ATR-FTIR spectroscopy (Bruker alpha-FT-IR spectroscopy with an ECO-ATR module) was used for scanning IR of the surface. The slide of the sample was applied on the surface of a ZnSe ATR crystal, which is acting as fibre optics. Perkin Elmer differential scanning calorimeter Pyris 1 was used for the determination of glass temperature Tg of solid polymers. The thermogram was recorded at a heating and cooling rate of 5°C/min. Gel Permeation Chromatography (GPC) Molecular weight (Mn) and Molecular weight distribution (Mw/Mn) of all polymers, copolymers and terpolymers were determined by gelpermeation chromatography with Knauer DMAc, polymer samples (6 g/L) were prepared with 2, 6-di-tert-butyl-4- methylphenol (BHT) as an internal standard. The measurements were performed at 30°C. UV-VIS spectra were recorded on UV-VIS Perkin Elmer lambda 45 spectrometer. For the estimation of photo-cross-linker content, 1 mol% of polymer solution in methanol for NIPAAm, DMAAm and their copolymers and terpolymers and THF was used for HEMA samples. UV Spectroscopy was also used for the determination of LCST of NIPAAm polymers, using metal covet stand and water cycle injected from the water bath (Julabo F12), with thermostat and cooling system. Over manual thermostat (TEMPERATUR-MESSGERAT MD 3040, BECKMANN +EGLE) was also used to adjust the actual temperature inside the solution. The polymer solution was 1 wt% in water. Spin coater model G3P-8 spin coat (specialty coating system, INT.) was used to prepare thin film (~ 200 nm dry thickness) on the glass substrate with 250 rpm for 30 sec and 2000 rpm for 140 sec. Thicker films were obtained by increasing the polymer concentration to 10% and using 1000 rpm. UV lamp (OSRAM, 100W, Hg lamp, wavelength>250 nm) equipped with an optical lens, a mirror and a double wall glass reactor connected to the water circulating thermostat were used for the photo-cross-linking process.
Synthesis of 2, 2-dimethyl-1, 3-dioxoylan-4-ylmethylacrylate
It has been achieved in two steps.
Step 1: Synthesis of isopropylideneglycerol: In 250 mL roundbottomed flask fitted with a reflux condenser and water trap to collect water. 36 g (0.62 mol) acetone, 30 g (0.44 mol) glycerol dissolved in 100 mL CHCl3. The mixture was stirred and 1.2 g (6.2 mol.) of p-toluenesulfonic acid was added. The reaction mixture was refluxed and stirred in an oil bath at 80℃. After, the time ended about 1.8 mL of water was collected. The reaction mixture allowed cooling and 1.3 g of sodium carbonate was added and refluxed again for 1/2 h. The precipitate was filtered and the solvent was evaporated under reduced pressure. It was colorless viscous liquid and 86% (lit: 82%) [2].
1H NMR (500 MHz, CDCl3): δ (ppm)=1.29 (s, 1H, 6-CH3), 1.34 (s, 1H, 6-CH3), 2.77 (br., 1H, OH), 3.52 (m, 1H, 2-CH2), 3.58 (m, 1H, 2CH2), 3.68 (m, 1H, 2CH2), 3.93 (m, 1H, 4-CH2), 4.13 (m, 1H, 3-CH).
13C-NMR (125 MHz, CDCl3): δ (ppm)=25.19 (1C, 1-CH3), 26.60 (1C, 1-CH3), 62.97 (1C, 5-CH2), 65.83 (1C, 3-CH2), 76.2 (1C, 4- CH), 109.27 (1C, 2-C).
IR (KBr): v (cm-1): 3400 (s) (OH), 2970(s) (CH-aliphatic), 1050 (s) (C-O-C ether).
Step 2: Synthesis of 2,2-dimethyl-1,3-dioxoylan-4-ylmethylacrylate: In 500 mL two-neck flask fitted with argon balloon. 37 g (0.28 mol) of (1) in 300 mL dry CH2Cl2 and allowed to stir strongly and 30.4 g (0.3 mol) of tea was added. The reaction mixture allowed cooling in an ice bath to 0℃-5℃. 22 g (0.24 mol) acryloyl chloride was added dropwise. The yellowish suspension solution was stirred at 0℃-5℃ for 1 h and then allowed to stir at RT for 2 d. The precipitate was filtered and the solvent was evaporated under reduced pressure. The product was extracted by 60 mL CH2Cl2 and washed three times with 300 mL distilled water. The organic phase was dried with MgSO4 overnight, then filtered and the solvent was evaporated under reduced pressure. The crude product was purified by vacuum distillation using an oil pump at 85℃-90℃ (10-2 bar). Colorless viscous liquid, 83%(lit: 85%).
1H NMR (500 MHz, CDCl3): δ (ppm)=1.32 (s, 3H, 1-CH3), 1.38 (s, 3H, 1-CH3), 3.73 (m, 1H, 5-CH2), 4.02 (m, 1H, 4-CH), 4.13 (m, 1H, 3-CH2), 4.19 (m, 1H, 3-CH2), 4.29 (m, 1H, 4-CH), 5.81 (dd, 2J=1,26 Hz, 3J=10.40 1H, 8a-CH), 6.10 (dd, 3J=10.72 Hz, 3J=17.34 Hz, 1H, 7-CH), 6.36 (dd, 2J=1.26, 3J=17.34 Hz, 1H, 8b- CH2).
13C-NMR (125 MHz, CDCl3): δ (ppm)=25.33 (1C, 1-CH3), 26.62 (1C, 1-CH3), 64.71 (2C, 5-CH2), 66.30 (2C, 3-CH2), 73.57 (1C, 4- CH), 109.79 (1C, 2-C), 127.96 (1C, 7-CH), 131.25 (2C, 8-CH2), 165.80 (1C, 6-C=O).
IR (KBr): v (cm-1): 2960(s) (CH-aliphatic), 1721(s) (C=O), 1630 (s) (C=C), 1180 (s) (C-O-C ether).
Synthesis of 4-acetylphenyl acrylate
In two neck flask fitted with argon balloon. 8 g (0.058 mol) of 4-hydroxyacetophenone was dissolved in 150 mL dry CH2Cl2 and allowed to stir strongly and 11.7 g (0.11 mol) of tea was added. The reaction mixture allowed cooling in an ice bath to 0℃-5℃. 5.24 g (0.058 mol) acryloyl chloride was added dropwise. The yellowish suspension was stirred at 5℃ for 1 h then, allowed to stir at RT overnight. The precipitate was filtered and the solvent was evaporated under reduced pressure. The product was extracted by CH2Cl2 and washed three times with distilled water, two with sodium carbonate and two with 0.1 M HCl. The organic phase was dried with MgSO4 overnight, then filtered and the solvent was removed under reduced pressure. Brownish solid, 78% (lit. 74%).
1H NMR (500 MHz, CDCl3): δ (ppm)=2.63 (s, 3H, 1-CH3), 6.09 (dd, 2J=0.73 Hz, 3J=10.3 Hz, 1H, 10a-CH), 6.36 (dd, 3J=Hz, 3J=Hz, 1 H, 9-CH), 6.76 (dd, 2J=Hz, 3J=Hz, 10b-CH), 7.26 (dd, 3J=12.54 Hz, 4J=2.56 Hz, 2H, 4,6-Ar-CH), 8.02 (dd, 3J=10.54 Hz, 4J=2.08 Hz, 2H, 3,7-Ar-CH).
13C-NMR (125 MHz, CDCl3): δ (ppm)=26.56 (1C, 11-CH3), 121.71 (2C, 5,7-Ar-CH), 127.57 (1C, 10-CH), 129.94 (2C, 4,8-Ar- CH), 133.21 (1C, 3-CH), 134.82 (1C, 11-C=C), 154.31 (1C, 6-Ar- C), 163.89 (1C, 9-C=O), 196.77 (1C, 2-C=O).
IR (KBr): v (cm-1): 2950-2970 (s) (CH2, CH3), 1727 (s) (2-C=O,), 1706 (s) (7-C=O,), 1600(s) (C=C), 784-885 (m) (Ar-CH).
Synthesis of Vanillin Acrylate or 4-formyl-2- methoxyphenylacrylate (VA)
In two neck flask fitted with argon balloon. 8 g (0.052 mol) was dissolved in 100 mL dry CH2Cl2 and allowed to stir strongly and 10.52 g (0.1 mol) of tea was added. The reaction mixture allowed cooling in an ice bath to 0℃-5℃. 5.4 g (0.059 mol) acryloyl chloride was added dropwise. The yellowish suspension was stirred at 5℃ for 1 h then, allowed to stir at RT overnight. The precipitate was filtered and the solvent was evaporated under reduced pressure. The product was extracted by CH2Cl2 and washed three times with distilled water, one with sodium carbonate and one with 0.1 M HCl. The organic phase was dried with MgSO4 overnight, then filtered and the product was distilled using an oil pump and at 100℃. Yellowish solid, 85%, mp 123℃ [3].
1H NMR (500 MHz, CDCl3): δ (ppm)=3.73 (s, 3H 1-CH3), 5.91 (dd, 2J=0.73 Hz, Hz 3J=10.52 Hz, 1H, 10a-CH), 6.20 (dd, 3J=10.45 Hz, 3J=17.36 Hz, 1 H, 9-CH), 6.51 (dd, 2J=0.73 Hz, 3J=17.36 Hz, 10b-CH), 7.13 (d, 2J=7.95 Hz 1H, 4-Ar-CH), 7.36 (dd, 3J=13.82 Hz, 4J=1.59, 1H, 6-Ar-CH, d, 4J=1.22 HZ, 5-Ar-CH), 9.96 (s, 1H, 7-CHO).
13C-NMR (125 MHz, CDCl3): δ (ppm)=55.91 (1C, 1-CH3), 111.09 (1C, 4-Ar-CH), 123.36 (1C, 5-Ar-CH), 124.24 (1C, 4-Ar-CH), 127.08 (1C, 10-CH), 133.16 (1C, 11-CH), 135.24 (1C, 6-Ar- C), 144.62 (1C, 3-Ar-CH), 151.91 (1C, 2-Ar-C), 163.22 (1C, 9- C=O), 190.99 (1C, 8-C=O).
IR (KBr): v (cm-1): 2950-2970 (s) (CH2, CH3), 2840 (m) (CH3), 1745 (s) (8-C=O, carbonyl), 1695 (s) (1-C=O, aldehyde), 1600(s) (C=C), 784-885 (m) (Ar-CH).
Synthesis of Polymers
Synthesis of poly (SKA-co-DMIA): In three round bottom flasks 5, 10 and 15 mol%, 0.125 g (0.383 mmol), 0.325 g (0.767 mmol) and 0.375 g (1.149 mmol) of acrylamide cross-linker DMIA respectively was added to 2.00 g (0.01 mol) SKA and AIBN as initiator. All mixtures were dissolved in 50 mL 1,4- dioxane. The reaction mixture was purged in argon for 20 min and then heated in an oil bath at 60℃ for 8 h. After cooling at room temperature and also in the refrigerator, the polymer was extracted in diethyl ether, at -50℃ and then the solvent was removed under reduced pressure to collect the viscous oil polymer. After this, the crude polymer was purified by dissolving in THF and re-precipitated in diethyl ether as a viscous oil.
1H NMR (CDCl3): δ (ppm)=1.29-1.46 (m, 6H, 10-2CH3), 1.47-1.78 (m, 3H, 6-CH, 5-CH2), 1.90-2.01 (m, 6H, 1-2CH3), 2.19-2.49 (m, 3H, 4-CH2, 3-CH), 3.63-3.86 (m, 2H, 7-CH2), 3.94-4.21 (m, 2H, 9-CH2), 4.22-4.39 (m, 1H, 8CH).
IR (KBr): v (cm-1): 2987-2940 (s) (CH-aliphatic), 1747 (s) (C=O), 1680 (s) (C=C).
Synthesis of terpolymer poly (NIPAAm-co-VA-co-APA): In three 100 mL round bottom flasks, mixtures of 5, 10, 15 mol% 0.167, 0.334, 0.501 g of (APA) was added to 10 mol% 0.362 g (VA) respectively and (0.0176 mol) 2.00 g NIPAAm in 50 mL 1,4-dioxane and 10-3 mol AIBN (total molar concentrations of monomers) was also added. The reaction mixture was purged in argon for 20 min and then heated in an oil bath at 70℃ for 8 h. After cooling at room temperature and also in a refrigerator, the polymer was precipitated in diethyl ether, at -60℃, then dissolved in THF and re-precipitated in diethyl ether to remove the unreacted monomers and impurities. The product was dried under reduced pressure as yellowish solid.
1H NMR (CDCl3): δ (ppm)=0.79-1.35 (m, 6H, 18-2CH3), 1.43-2.46 (m, 9H, 5, 7, 14-CH2, 6, 8, 15-CH), 2.47-2.66 (m, 3H, 1-CH3), 3.78-4.19 (m, 1H, 17-CH), 5.88-6.72 (m, 1H, 16-NH), 7.07-7.23 (m, 3H, 3, 9-3CH-Ar.), 7.38-7.59 (m, 2H, 10, 11-2CH), 7.84-8.05 (m, 2H, 2-2CH), 9.86-10.04 (m, 1H, 13-CHO).
IR (KBr): v (cm-1): 3363 (N-H), 3069 and 2978 (CH-aliphatic), 1762 (s) (C=O, carbonyl), 1690 (s) (C=O, aldehyde), 1570 (s) (C=O amide), 1104 (m) (CH3), 860 (m) (CH-Ar).
Preparation of Hydrogel Thin Film
Fabrication of gel thin ilms based on poly (SKA-co-DMIA): A slide of LaSFN9 glass (2 cm. H × 2 cm. W) was cleaned by pure ethanol and dried under reduced pressure. Pure gold (99.9999%, Aldrich, Germany) has been evaporated to deposit 45 nm-50 nm film thickness and then, the gold was immersed in a solution of 5 mM 3-(3,4-dimethyl-2,5-dioxo-2,5-dihydropyrrol- yl)-propyl ester (DMITAc). 10 wt% poly (SKA-co-DMIA) polymer solution with 0.0005 wt% thioxanthone in distilled cyclohexanone was spin-coated over LaSFN9+DMITAc (2000 rpm). The coated slide has been exposed to UV-irradiation through UV-lamp (300 nm-430 nm). The cyclodimerization (2+2) between dimethyl maleimide groups and the photocross- linking was formed.
Ring-opening of SKA to DHPA: In 100 ml flat bottom flask fitted by reflux condenser, the spin-coated glass slide has been prepared in step A was immersed in a mixture of 15 ml THF and 15 mL glacial acetic acid. The mixture was refluxed for 5 h at 90℃. The slide was removed and washed by THF, distilled water and then dried under vacuum overnight. ATR was used to see the change in surface, SPR-OW was used to measure thickness and refractive index in the dry case; moreover, the swelling properties were discussed.
IR v (cm-1): 3500-3900 (s, br. multi-peaks) (OH, CH-aliphatic), 1672-1738 (br. multi-peaks) (C=O), 1570-1588 (s, multi-peak) (C=C).
Grafting of hydrogel thin film poly (DHPA-co-DMIA)-g-poly (NIPAAm-co-APA-co-VA): In 50 mL round-bottomed flask fitted with a reflux condenser. A slid coated by poly (DHPA-Co- DMIA) was immersed in a solution of poly (NIPAAm-co-APAco- VA) in 25 mL CHCl3. 0.1 g (0.58 mmol) of p-toluenesulfonic acid was also added. The solution with a glass slide was refluxed in an oil bath at 80℃. After that, the reaction mixture allowed cooling and 0.2 g of sodium carbonate was added and refluxed again for 30 min. The slide was removed and washed by THF followed by DI water, then dried under vacuum for 2 h. ATR was used to investigate the change in surface, SPR-OW was used to measure thickness and refractive index in the dry case and the swelling properties were discussed.
IR v (cm-1): 3500-3700 (m, br. multi-peaks) (NH, CH-aliphatic), 2350 (s) (O-C-O), 1668-1730 (br. multi-peaks) (C=O), 1570-1588 (s, multi-peak) (C=C), 720 (m) (CH-Ar).
Swelling Measurements
The film thickness for each layer and swelling properties has been scanned by surface plasmon resonance with an optical waveguide. The change in refractive indexes RI (θ) was used to intemperate the degree of swelling. Knoll, et al. discuss the relation between waveguide and film thickness demonstrated indirect relation as the higher waveguide modes with the lower incident angles and vice versa. However, the plasmon minimum can be displaced from ~ 65.5° to ~ 68° indicating the phase separation from swollen to collapsed by simulations and the fresnel calculations the relation of volume degree of swelling or refractive index with temperature has been used for determination of lower critical solution temperature of the hydrogel [4].
Fabrication of Monomers
The present work utilizes the preparation of three hydrophobic monomers with and without functionality as described in Figure 1.
2, 2-dimethyl-1, 3-dioxoylan-4-yl-methylacrylate (Solketal Acrylate) (SKA) has been prepared in two steps, the first is the formation of isopropylideneglyceol by the reaction of acetone and glycerol in acid media the product evaluated by 1H NMR showed the presence of 2CH3 groups at δ=1.27 and 1.34 ppm and broad peak at δ=2.75 ppm for OH group, 13C and FTIR all was in logic state with the chemical structure. The next step is the preparation of final product 2, 2-dimethyl-1, 3- dioxoylan-4-yl-methylacrylate by reacting with acryloyl chloride in the presence of triethylamine as the basic condition, the product was deduced by 1H NMR, 13C and FTIR represented the formation of 3H of vinyl group at δ=5.8, 6.0 and 6.3 ppm with respect to C=C at δ=127 and 131 ppm and 1660 cm-1. The next monomer is 4-acetylphenyl acrylate by reacting of 4-hydroxyacteophenone with acryloyl chloride in basic media such as triethylamine. The product was evaluated (1HNMR, 13C and FTIR) demonstrated the formation of 3H vinyl group and disappearance of hydroxyl group δ=6.00, 6.33 and 6.66 ppm, δ=127, and 134 ppm and v=1635 cm-1.
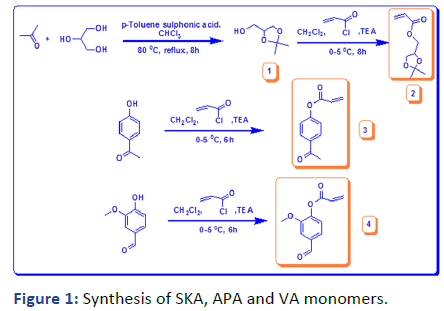
Figure 1: Synthesis of SKA, APA and VA monomers.
The last monomer was prepared from vanillin in one-step reaction between vanillin and acryloyl chloride, the product was investigated by (1HNMR, 13C and FTIR) indicating the formation of vinyl group and the presence of aldehyde group at δ=9.79 ppm, δ=190.88 and v=1735 cm-1.
Fabrication of Polymers Gel Thin Films
Here, we are concerned with two kinds of polymers. The copolymerization polymerization of SKA with different molar ratios of DMIAAm photo-cross-linker has been done by the free radical polymerization in a solution using AIBN as an initiator as Figure 2. The fabricated polymers were evaluated by 1H NMR and IR; overall analyses were in good agreement with the chemical structure. The actual concentration of each monomer in the polymer chain was calculated from the 1H NMR integration of 6H, 2CH3 of SKA at δ=1.35 to 1.41 ppm or 5H, 2CH2 and CH at δ=3.74 to 4.3 ppm with 4H, 2CH2 DMIAAm at δ=1.96 ppm as shown in Table 1. The terpolymers of NIPAAm, VA and APA with different molar ratios of APA 5, 10 and 15 and 10 molar concentration of VA have been also prepared by free radical polymerization in solution and AIBN as initiator as shown in Figure 2.
The presence of two functional groups demonstrated in ketone and aldehyde for APA and VA respectively encourage the formation of other chemical reactions as will discuss in the next step. The chemical structures have been investigated by 1HNMR and IR and all showed good agreement. The concentrations of each monomer were determined by the 1HNMR integration of specific protons at δ=4.1 ppm 1H, CH for NIPAAm and at δ=2.58 ppm 3H, CH3 APA and at δ=9.97 ppm 1H, CHO, VA as shown in Table 1. FTIR of solid monomers and polymer with dry KBr illustrated some specific peaks related to functional groups (C=O stretch) ester at 1740 cm-1 and (C=C stretch) vinyl at 1665 cm-1 (Figures 3-13).
The surface of the gel was exposed to the chemical reaction and ring-opening of SKA then ring closure with APA and crosslinking, the process has been investigated by applying the surface to ZnSe ATR crystal. Figure 14 showed the formation of OH groups at 3500-3900 (s) multi-peaks, otherwise, the disappearance of these peaks after cross-linking with APA as shown in Figure 15 [5].
| Polymer | Yield% | Composition DMIA (mol%) | Mna (g/mol) 104 | Db | Tg°Cc | Tc°Cd | |
|---|---|---|---|---|---|---|---|
| 1HNMR | UV | ||||||
| 6a-05 | 65 | 3.2 | 3.55 | 1.89 | 1.83 | -23 | - |
| 6b-10 | 62 | 7.8 | 7.9 | 2.49 | 1.86 | -28 | - |
| 6c-15 | 57 | 14.08 | 14.4 | 0.49 | 2.1 | -37 | - |
| 1HNMR | |||||||
| APA | VA | ||||||
| 7a-05-10 | - | 4.55 | 8.9 | 1.32 | 1.96 | 123 | 18 |
| 7b-10-10 | - | 9.86 | 8.88 | 1.28 | 2.1 | 117 | 15 |
| 7c-15-10 | - | 14.65 | 8.75 | 1.19 | 2.31 | 108 | 13 |
Table 1: Yield, composition, molecular weight, polydisperisity and glass temperature of poly (SKA-co-DMIA).
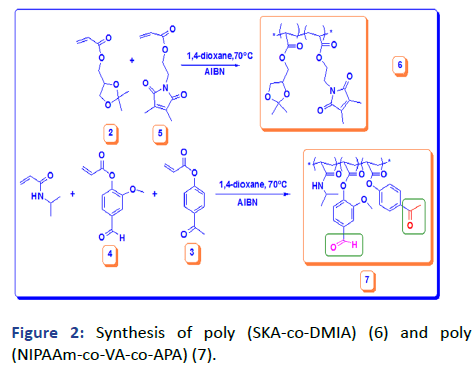
Figure 2: Synthesis of poly (SKA-co-DMIA) (6) and poly (NIPAAm-co-VA-co-APA) (7).
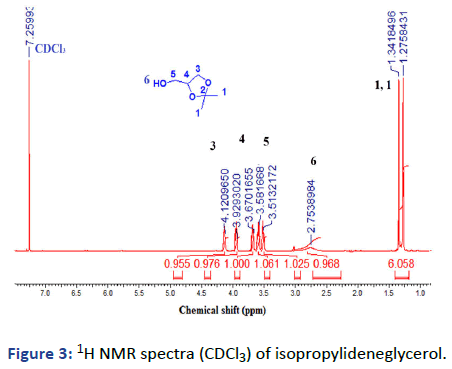
Figure 3: 1H NMR spectra (CDCl3) of isopropylideneglycerol.
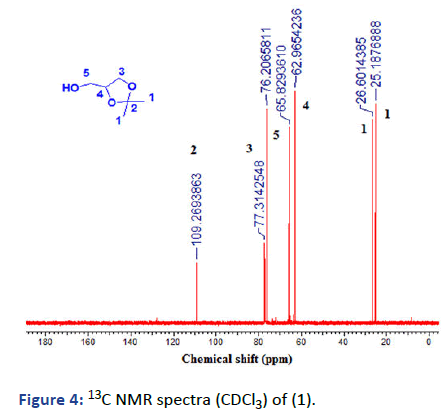
Figure 4: 13C NMR spectra (CDCl3) of (1).
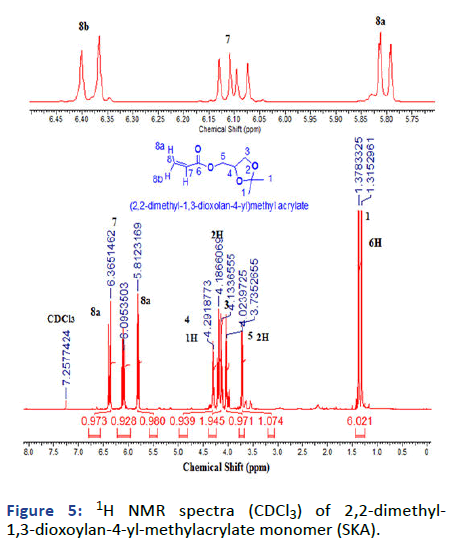
Figure 5: 1H NMR spectra (CDCl3) of 2,2-dimethyl- 1,3-dioxoylan-4-yl-methylacrylate monomer (SKA).
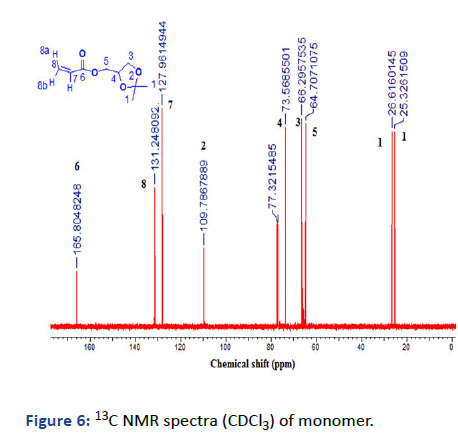
Figure 6: 13C NMR spectra (CDCl3) of monomer.
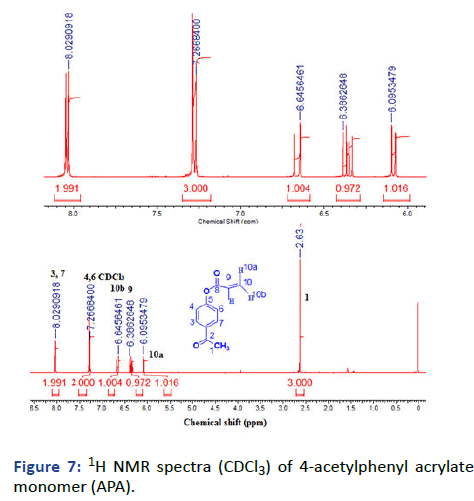
Figure 7: 1H NMR spectra (CDCl3) of 4-acetylphenyl acrylate monomer (APA).
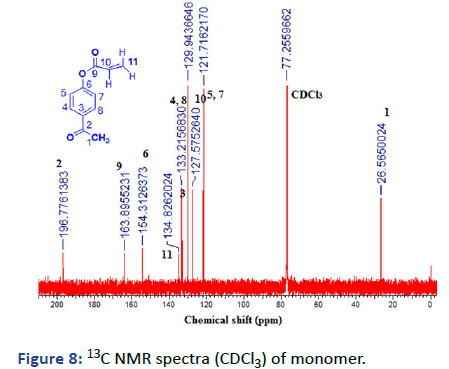
Figure 8: 13C NMR spectra (CDCl3) of monomer.
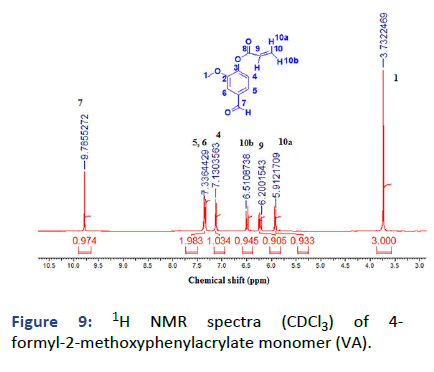
Figure 9: 1H NMR spectra (CDCl3) of 4- formyl-2-methoxyphenylacrylate monomer (VA).
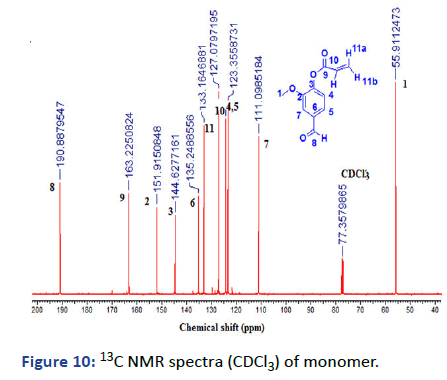
Figure 10: 13C NMR spectra (CDCl3) of monomer.
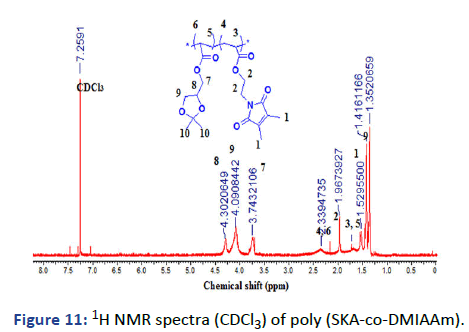
Figure 11: 1H NMR spectra (CDCl3) of poly (SKA-co-DMIAAm).
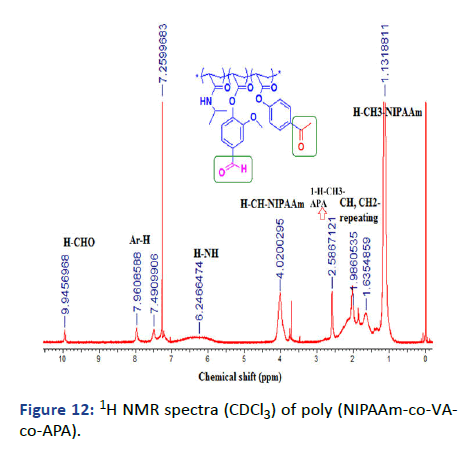
Figure 12: 1H NMR spectra (CDCl3) of poly (NIPAAm-co-VAco- APA).
Figure 13: FT-IR of monomers and polymers.
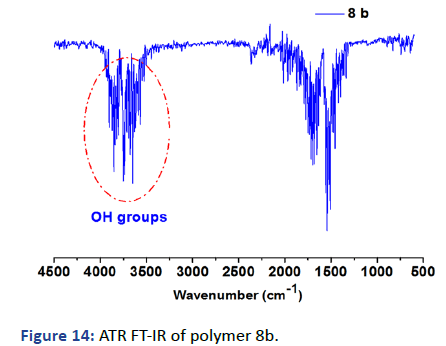
Figure 14: ATR FT-IR of polymer 8b.

Figure 15: ATR FTIR of polymer 8c.
Polymer Characterizations
The molecular weight of polymers has been recorded by Gel Permeation Chromatography (GPC) using Dimethylacetamide (DMAAm) as an eluent and Polystyrene (PS) column; the concentration used 6 g/L. Figure 16 showed the relation between the weight average molecular weight with log (M) and demonstrated one peak for each polymer which emphasis the formation of polymer and the disappearance of impurities and small molecules like monomer. The other observation has been pointed in the polydispersity showed a decrease in D by increasing the photocross- linker in polymer 6 and VA and APA for polymer 7 interpreted the free radical polymerization and the formation of copolymers and terpolymers for 6 and 7 respectively. The glass transition temperature Tg was measured by Differential Scanning Calorimetry (DSC) and has been recorded on thermogram as the second-order change in the heat flow. The DSC thermograms represent decreasing in Tg -23℃, -28℃ and -37℃ of polymers 6a-05, 6a-10 and 6a-15 respectively due to the high hydrophobicity of both SKA and DMIAAm in the polymer main chain which decreases stiffness and increases flexibility (Figure 17) [6]. On the other hand, polymers 6a-05, 6a-10 and 6a-15 copolymerized with N-isopropyacryamide have higher glass transition temperature Tg attributed to the presence amide group which increases the hydrophilicity in the polymer chain further increase stiffness and decrease flexibility (Figure 18). The phase separation demonstrated in the lower critical solution temperature of temperature-responsive polymers 7a-05, 7a-10 and 7a-15 showed the lower value of Tc rather than N-isopropyacryamide homopolymer. Figure 19 showed the relation between temperature and transmittance of polymers 7a-05, 7a-10 and 7a-15 and the Tc was determined at the inflection point exhibited 18℃, 15℃ and 13℃ respectively; the lowest value was detected for the higher molar concentration of APA and VA for its higher hydrophobicity which faster the phase separation in solution [7].
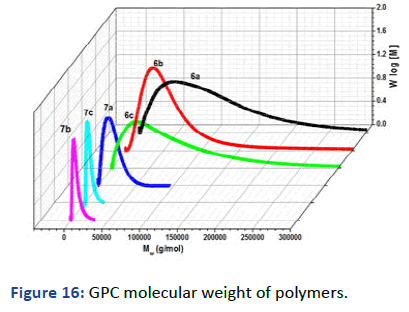
Figure 16: GPC molecular weight of polymers.
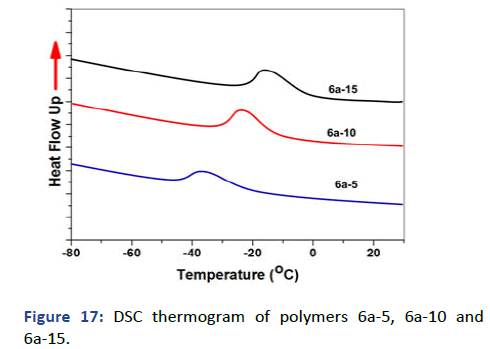
Figure 17: DSC thermogram of polymers 6a-5, 6a-10 and 6a-15.
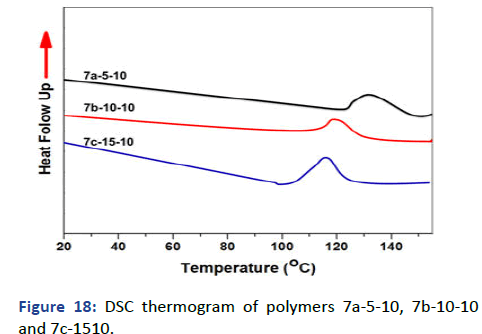
Figure 18: DSC thermogram of polymers 7a-5-10, 7b-10-10 and 7c-1510.
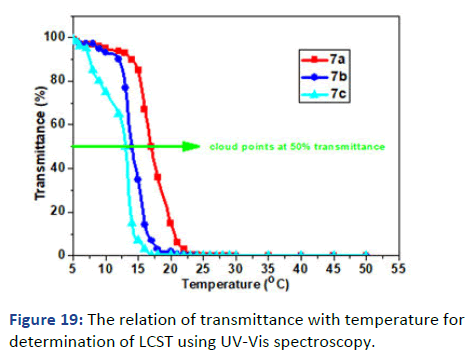
Figure 19: The relation of transmittance with temperature for determination of LCST using UV-Vis spectroscopy.
Gel Thin Films
The aim of this study is the modification of the surface of the hydrophobic layer based on PSKA photo-cross-linked gel film. The modification process involved three steps; each step was described by SPR/OWS for both dry and swollen states [8].
Formation of the hydrophobic gel layer: The formation of PSKA gel thin film was achieved using poly (SKA-Co-DMIA) 10 mol% of DMIA and 10 wt% of the polymer solution in cyclohexanone at 2000 rpm. SPR/OWS scan for the dry film showed the absence of Attenuated Total Reflection (ATR) and the presence of waveguide at 250 which indicates the formation of the homogenous film. Fresnel calculations of the dry thickness showed 264 nm and 1.473 Refractive Index Unit (RIU) (Table 2).
The swelling was measured by SPR/OW and using DI water. The scan of theta (θ) vs. RI for variations of temperature has been done. Fresnel calculations have been used for the determination of the refractive index that used for the determination of the volume degree of swelling. Figure 22 showed the relation between volume degree of swelling and refractive index with temperature, the surface demonstrated unaffectedness to temperature [9].
| Dry thickness (nm) | Refractive index (n2=ε) | n |
|---|---|---|
| 264 | 2.17 | 1.473 |
| 250 | 2.18 | 1.476 |
| 320 | 2.15 | 1.466 |
Table 2: Illustrate dielectric and refractive indices.
Conversion to the hydrophilic gel layer: The hydrophobic gel layer has been converted to the hydrophilic one by the ringopening of SKA as described in the experimental section. The chemical structure was investigated by ATR administrated logic results. The overall process has been schematically drawn in Figure 20. The surface has been investigated by SPR/OWS for dry and swell film thickness. Fresnel calculations for dry layer showed a thickness of 250 nm and ~1.476 RIU with waveguide at θ=23 as shown in Figure 21.
The swelling was measured by SPR/OW and using DI water. The scan of theta (θ) vs. RI with variations of temperature has been performed. The other observations are increasing in volume degree of swelling to be ~1.6 to ~1.7 and refractive indices to be ~1.39 RIU to ~1.38 RIU as raising the temperature, indicating anisotropic behavior. Figure 22 describes the change in the volume degree of swelling and refractive index with temperature as an independent and demonstrates anisotropic behavior [10].
Formation of the temperature-responsive gel layer: In the previous step, the gel layer has been converted to hydrophilic and free hydroxyl groups were created. APA polymer solution was dissolved in cyclohexanone and then coated to the gel layer that further chemically cross-linked as discussed in the experimental section.
The SPR/OWS scan for the dry state showed the presence of waveguide mode with ATR. Fresnel calculations for dry single layer showed a thickness of 320 nm and ~1.466 RIU as shown in Figure 23 indicated the presence of the additional thin film. The swelling was measured by SPR/OW and using DI water [11]. The scan of theta (θ) vs. Refractive Index (RI) with variations of temperature has been achieved.
Figure 24 exhibited swelling behavior as a change in RI (θ) with temperature change. The scan indicated three important features;
• The plasmon minima shifted from ~75 to ~77 with raising
the temperature.
• The volume degree of the swelling has been higher than
the previous step.
• The refractive index was lower than the previous step.
The lower critical solution temperature of the upper layer was detected by the relation of volume degree of swelling and refractive index with temperature. It is difficult to detect the change due to the effect of the hydrophobic layer that covers and protects the sensitivity of the upper layer [12]. However, the higher protection of the hydrophobic layer still NIPAAm has the affection and demonstrated LCST (Tc) at approximately 20℃ at the inflection point as shown in Figures 25 and 26.
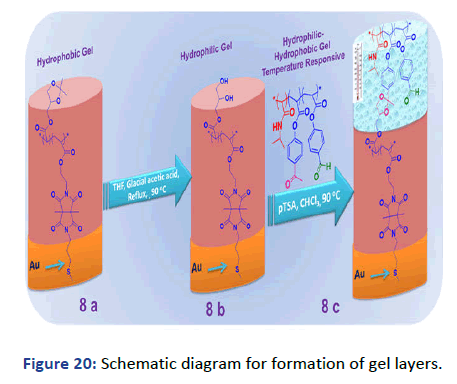
Figure 20: Schematic diagram for formation of gel layers.
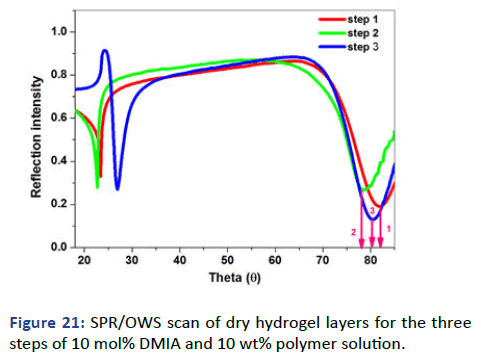
Figure 21: SPR/OWS scan of dry hydrogel layers for the three steps of 10 mol% DMIA and 10 wt% polymer solution.
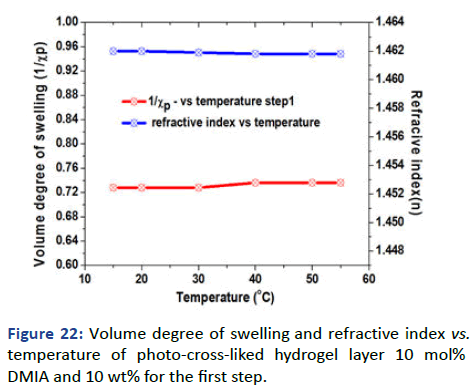
Figure 22: Volume degree of swelling and refractive index vs. temperature of photo-cross-liked hydrogel layer 10 mol% DMIA and 10 wt% for the first step.
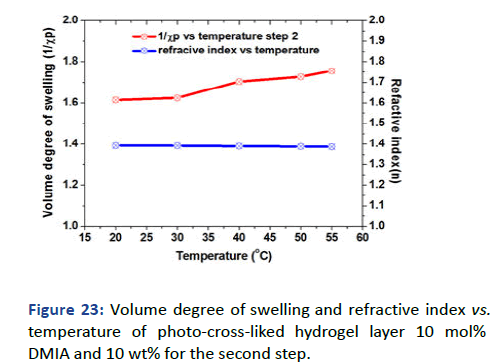
Figure 23: Volume degree of swelling and refractive index vs. temperature of photo-cross-liked hydrogel layer 10 mol% DMIA and 10 wt% for the second step.
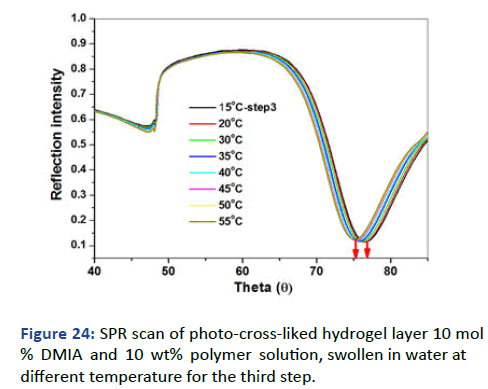
Figure 24: SPR scan of photo-cross-liked hydrogel layer 10 mol % DMIA and 10 wt% polymer solution, swollen in water at different temperature for the third step.
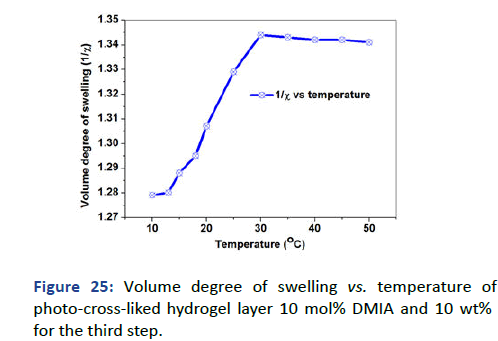
Figure 25: Volume degree of swelling vs. temperature of photo-cross-liked hydrogel layer 10 mol% DMIA and 10 wt% for the third step.
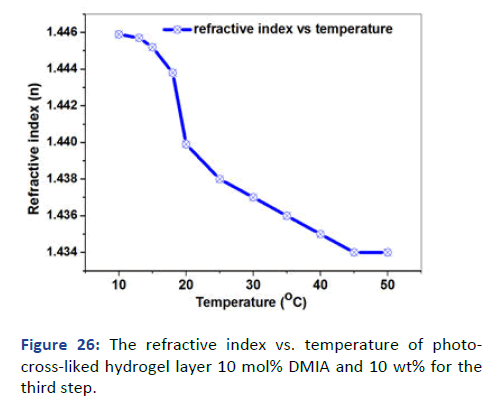
Figure 26: The refractive index vs. temperature of photocross- liked hydrogel layer 10 mol% DMIA and 10 wt% for the third step.
The present work can be concluded in three stages. First of all, the fabrication of new acrylate monomers that acts as functional and hydrophobic monomers. The next step was the free radical polymerization of two kinds of copolymers. The effect of the hydrophobic monomer in N-isopropylacrylamide was discussed and demonstrated lower values of lower critical solution temperature by increasing the molar concentration of the hydrophobic monomers.
The last step was the formation of photo-cross-linked solketal acrylate gel over gold. Surface plasmon resonance with optical waveguide was used for the determination of film thickness of the dry and swollen gel. It had not any response with temperature as the change of volume degree of swelling or refractive index with temperature. The surface of SKA was modified by ring-opening and formation of hydroxyl groups that facilitate increasing of hydrophilicity of the polymer gel.
SPR/OW was measured for dry and swollen gel. The free hydroxyl groups on the gel surface were grafted by poly (NIPAAm-co-VA-co-APA) and SPR/OW was used; the gel surface showed thermo-responsive behavior and the lower critical solution temperature was detected as the change of volume degree of swelling or refractive index with temperature. This study is the beginning of the fabrication of gel in the biological industry and bioseparation through the formation of gel vessel for attaching and releasing of biomolecules.
The authors are gratefully acknowledged to Egyptian culture and missions and the Deutscher Akademischer Austauch (DAAD) for financial assistance during the post-doctor work in Germany of Momen S.A. Abdelaty.
The author declares no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Abdelaty MSA (2024) Photo-Cross-Linked Solketal Acrylate Gel Multilayer Thin Films: Fabrication, Characterization and Comprehensive Study Surface Plasmon Resonance/Optical Waveguide. J Polymer Sci. 9:11.
Copyright: © 2024 Abdelaty MSA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.