- (2013) Volume 14, Issue 2
Metesh Nalin Acharya1, Nikolaos Panagiotopoulos1, Patrizia Cohen2, Raida Ahmad2, Long R Jiao1
Departments of 1Surgery and Cancer and 2Histopathology, Hammersmith Hospital. London, United Kingdom
Received October 26th, 2012 – Accepted November 27th, 2012
Context Signet-ring cell carcinoma (SRCC) of the ampulla of Vater is a very rare clinical entity, which is infrequently reported in medical literature. Case report A 78-year-old woman was admitted with jaundice, pruritus and postprandial vomiting. Abdominal ultrasound and computed tomography scanning demonstrated gross dilatation of the common bile and pancreatic ducts with gallbladder calculi. Endoscopic retrograde cholangiopancreatography suggested a duodenal tumour at the ampulla. The patient underwent Whipple’s procedure with cholecystectomy. Immunohistopathological examination confirmed poorly-differentiated SRCC of the ampulla of Vater. The tumour had infiltrated the duodenal muscularis propria and pancreatic parenchyma, but local lymph nodes were clear (T3N0M0). The patient was disease-free at 6-month follow-up. Conclusions We here report a case of poorly-differentiated SRCC of the Ampulla of Vater. The majority of patients with such tumours undergo pancreaticoduodenectomy, which affords good outcomes in early disease. However, owing to the rarity of cases, the exact prognosis of ampullary SRCC remains as yet undetermined.
Ampulla of Vater; Carcinoma, Signet Ring Cell; Pancreas
SRCC: signet-ring cell carcinoma
Most tumours of the ampulla of Vater are welldifferentiated adenocarcinomas. Signet-ring cell carcinoma (SRCC) is a very rare histological variant found at this site, being more common in the stomach than elsewhere in the digestive system [1]. Including the original report by Sekoguchi et al. in 1979 [2], 31 previous cases of ampullary SRCC have been mentioned in medical literature. We here present an elderly patient who underwent Whipple’s procedure, in whom poorly-differentiated ampullary SRCC without nodal metastasis was confirmed on histological examination of the resected specimen.
A 78-year-old woman presented to our institution with a three-week history of tiredness, postprandial vomiting, jaundice and pruritus. She additionally reported oily stools. Her past medical history included hypothyroidism, chronic cystitis and a left knee replacement. She was visibly icteric, but physical examination was otherwise unremarkable, without any abdominal tenderness or palpable breast masses. Routine laboratory investigations revealed elevated alanine transaminase 106 IU/L (reference range: 0-40 IU/L), alkaline phosphatase 730 IU/L (reference range: 30-130 IU/L), and bilirubin 182 μmol/L (reference range: 0-17 μmol/L), with normal inflammatory markers.
Abdominal ultrasound demonstrated gross dilatation of the common bile and pancreatic ducts, and gallbladder, the latter containing several small calculi. A 2 cm soft tissue mass was suggested at the level of the ampulla of Vater. Subsequent staging CT scan confirmed the marked common bile and pancreatic duct dilatation. However, no definite ampullary mass lesion was identified; a bulky pancreatic head was noted, with a homogenous texture similar to the rest of the pancreas. There was no ultrasound or CT evidence of local spread, vascular encasement or distant metastatic disease. Endoscopic retrograde cholangiopancreatography (ERCP) suggested a duodenal tumour at the ampulla, but a stent could not be placed across the stricture and biopsy was not taken.
The patient underwent Whipple’s resection and cholecystectomy. At operation, a large mass was found at the head of the pancreas, involving the first and second parts of the duodenum, and invading into the hepatic aspect of the transverse colonic mesentery. Following an uncomplicated post-operative course, the patient was discharged home. She did not receive adjuvant chemoradiotherapy, and had no evidence of recurrence at six-month follow-up.
On histological analysis, an ill-defined lesion measuring 12 mm in maximum dimension was noted near the ampulla, extending to its anterior margin (Figure 1). The tumour measured 30 mm in maximum dimension within the duodenal submucosa, from where it ulcerated into the duodenal mucosa, surrounding blood vessels, the ampullary duct and the common bile duct (Figure 2). It infiltrated the duodenal muscularis propria and extended into the pancreatic parenchyma, reaching 16 mm from the superior mesenteric vein. Although there was extensive perineural invasion with infiltration of the duodenal mucosal and submucosal lymphatics, the pancreatic resection margin was not involved, and 0/13 peripancreatic and 0/10 greater curve lymph nodes were all free of tumour (T3N0M0). Microscopic appearances showed a poorlydifferentiated signet-ring cell carcinoma, composed entirely of round cells containing intra-cytoplasmic mucin (Figure 3). Immunohistochemical staining was strongly positive for cytokeratin (CK) 7, CK20, CK8/18, CK19, CEA mono, CA 19-9, CA 125, MUC1 and MUC5AC. Tumour cells showed no expression for MUC2, MUC6, SMAD4, CDX2, ER and PgR. This immunohistochemical staining pattern is highly suggestive of signet-ring cell carcinoma of pancreatobiliary origin [3].
Adenocarcinoma of the ampulla of Vater is a rare clinical entity, occurring in less than six cases per million people annually [4]. It accounts for 0.2% of all gastrointestinal and 6% of periampullary malignancies [4, 5]. SRCC represents a variant of adenocarcinoma, characterised by the presence of greater than 50% signet-ring cells with intra-cytoplasmic mucin, and typically eccentrically-located crescent-shaped nuclei [4]. It may arise in any organ, especially in the stomach where it comprises 15-30% of all gastric cancers [5].
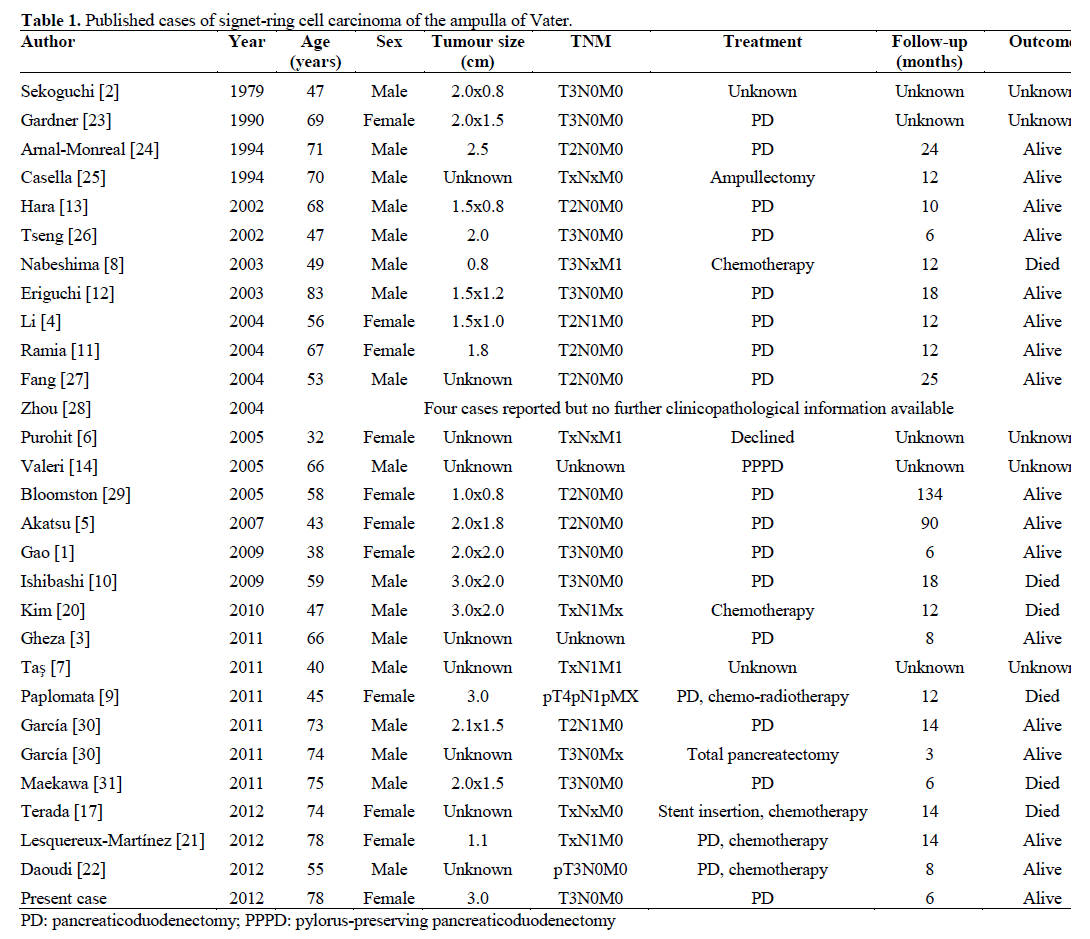
We here present the 32nd report of SRCC of the ampulla of Vater in medical literature (Table 1). From available demographic information, the 30 preceding cases included 16 males and 9 females aged between 32 and 83 years of age. One patient presented with T4 disease, ten patients with T3 disease and eight with T2 disease. Diagnostic imaging detected metastatic lung, liver and bone disease in one patient [6], and multiple pulmonary metastatic deposits in another [7]. Disseminated carcinomatosis was noted on presentation in a Japanese patient [8], whilst leptomeningeal metastases were detected in another patient following adjuvant chemotherapy for ampullary SRCC [9].
The rarity of ampullary SRCC is compounded by its unclear histological genesis. Since SRCC is more frequently encountered within gastric epithelium, it has been suggested that this tumour may arise from ectopic gastric mucosa situated at the ampulla [10, 11]. Another theory proposes that SRCC originates from gastric-type metaplasia occurring as a protective response to increased acidity, a recognised phenomenon in peptic ulcer disease [10, 11]. Although peri-tumoural ectopic gastric mucosa was found in two previous patients with SRCC, none was found in our patient, who also did not suffer peptic ulcer disease.
Immunohistochemical staining patterns allowing further classification of ampullary SRCC to a pancreatobiliary- or intestinal-type have been described [3]. Expression of CK7, along with negativity for CK20, CDX-2 and MUC2 signifies pancreatobiliarytype SRCC, and vice-versa. Based on these staining patterns, tumour cells in the present case were suggestive of a pancreatobiliary-type origin, which is associated with a less favourable outcome [3, 12].
It is known that SRCC elsewhere in the gastrointestinal tract has a poor prognosis [1, 3, 5]. Similarly, poorlydifferentiated ampullary adenocarcinoma usually signifies advanced-stage disease, with the occurrence of nodal metastasis and pancreatic invasion [5]. Although our patient’s tumour featured poorlydifferentiated signet-ring cells, there was no lymph node involvement at presentation. Indeed, prognosis seems closely related to the extent of neural invasion and nodal metastasis at the time of surgery [3]. Nevertheless, prognosis is difficult to ascertain in ampullary SRCC, compared to ampullary adenocarcinoma, owing to the limited number of reports so far.
The majority of patients in previous cases of ampullary SRCC underwent pancreaticoduodenectomy, occasionally with extended lymphadenectomy and/or partial gastrectomy. This radical approach facilitates lymph node dissection in advanced disease states, but a pylorus-preserving technique, which has been utilised in three previous cases of ampullary SRCC [9, 13, 14], may be more applicable in early disease, where curability is balanced by a more moderate resection. Although currently a controversial strategy, pyloruspreserving pancreaticoduodenectomy for suspected peri-ampullary malignancy has been shown to be comparable to classical Whipple’s operation, in terms of operating time, intra-operative blood loss, complications such as delayed gastric emptying, as well as disease-free survival [15, 16]. Nevertheless, our patient recovered in an uneventful manner following Whipple’s resection. Interestingly, one group successfully performed ampullectomy using a transduodenal approach for ampullary SRCC, with no evidence of disease at twelve month follow-up; this technique has not been utilised since. Endoscopic stent insertion has also been reported as a conservative treatment [17].
We performed a classical Whipple’s procedure to maximise curative potential, since pre-operative histological diagnosis was not available. On account of negative resection margins and absence of nodal involvement, post-operative chemotherapy was not deemed necessary.
Chemoradiotherapy based on 5-fluorouracil has been employed as an adjunctive treatment modality following curative resection of ampullary adenocarcinomas. However, there is debate as to whether this actually affords a statistically significant survival benefit, since many patients develop metastatic disease [18, 19]. Presently, no established adjuvant chemotherapeutic regimen exists specifically for ampullary SRCC, although documented antineoplastic agents have been used in five cases.
Nabeshima et al. treated a 49-year-old male patient with ampullary SRCC and secondary bone metastasis (pT3NxM1) with 5-fluorouracil and leucovorin, reporting increased survival and quality of life [8]. Another 45-year-old male patient with pT4pN1pMX ampullary SRCC received six months of adjuvant gemcitabine and oxaliplatin, but three months subsequently developed leptomeningeal metastases, with a poor response to intrathecal methotrexate [9]. In Kim et al.’s report, a 47-year-old male patient, found to have multiple nodal metastases at operation, was conservatively managed with biliary stenting and titanium silicate/cisplatin chemotherapy, but died one year post-diagnosis [20]. In contrast, Lesquereux- Martínez et al. treated a 78-year-old female patient with ampullary SRCC and minimal neoplastic nodal infiltration (1/21 lymph nodes) with adjuvant gemcitabine, finding her disease-free at 14 months [21]. In Douadi et al.’s report, a 55-year-old male patient with pT3N0M0 disease received six cycles of adjuvant gemcitabine/cisplatin, without disease recurrence at eight months [22].
Thus, adjuvant chemotherapy may be appropriate in the treatment of ampullary SRCC associated with locoregional metastasis. However, its role in node-negative and distant metastatic disease remains as yet undetermined and, from the case reports evaluated, consensus regarding the optimum chemotherapeutic regimen is lacking.
In conclusion, we here report a very rare case of poorly-differentiated ampullary carcinoma with signetring features, but without distant metastatic disease. We believe the tumour arose from pancreatobiliary epithelium, according to immunohistochemical staining patterns. Whilst pancreaticoduodenectomy can afford a good outcome in early, node-negative SRCC, the longterm prognosis remains uncertain.
The authors declare they have no conflicts of interest to disclose
No external sources of funding