- (2009) Volume 10, Issue 4
Deepak Kumar Singh1, Aiman Haider1, Medha Tatke1, Pankaj Kumar2, Pramod Kumar Mishra2
Departments of 1Pathology and 2Gastrointestinal Surgery, Govind Ballabh Pant Hospital. New Delhi, India
Received February 26th, 2009 - Accepted May 27th, 2009
A 45-year-old female presented with upper abdominal pain. Ultrasound and CECT both showed a mass in the pancreatic head and uncinate process suggestive of carcinoma of the head of the pancreas. A Whipple’s pancreaticoduodenectomy was carried out. Microscopic examination showed numerous epithelioid cell granulomas in the pancreas infiltrating the duodenal mucosa. No evidence of malignancy was seen. Acid fast bacilli were positive. The patient was started on anti-tubercular therapy. Cases of isolated pancreatic tuberculosis are very rare and can mimic pancreatic carcinoma clinically and radiologically. A differential diagnosis of pancreatic tuberculosis must be made in a patient presenting with a pancreatic mass and the patient must be thoroughly investigated to confirm or rule out malignancy or tuberculosis. We herein present a case of primary pancreatic tuberculosis and discuss the clinicopathological features of this condition. We have also proposed an algorithm for the management of patients presenting with a pancreatic mass and a clinical suspicion of pancreatic tuberculosis.
Ampulla of Vater; Carcinoma; Pancreas; Pancreaticoduodenectomy; Tuberculosis
The most common site for abdominal tuberculosis is the ileocecal area [1]. Cases of isolated pancreatic tuberculosis with the pancreas being the only site of involvement are very rare. We present a case of pancreatic tuberculosis which mimicked pancreatic carcinoma clinically and radiologically. Out of 157 cases who underwent Whipple’s pancreaticoduodenectomy in our institute in 2008 subsequent to a clinical diagnosis of ampullary carcinoma, the present case was the only one with isolated pancreatic tuberculosis.
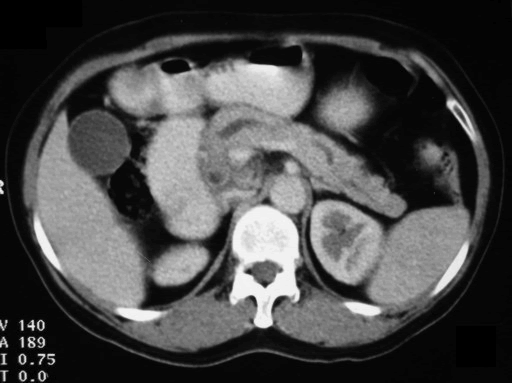
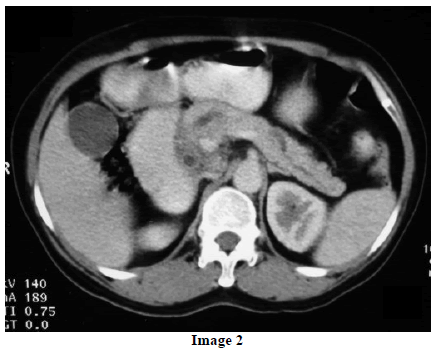
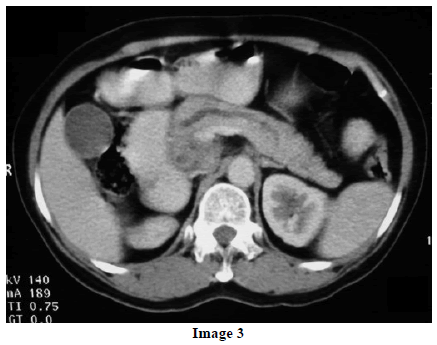
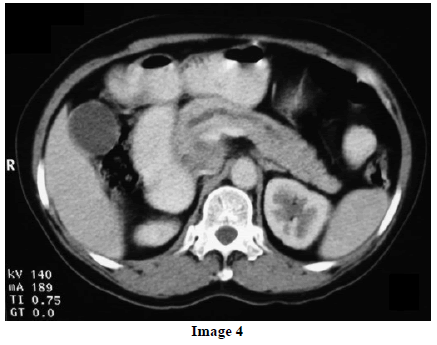
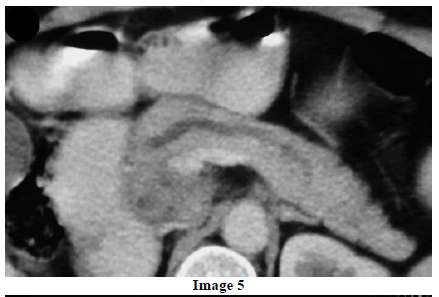
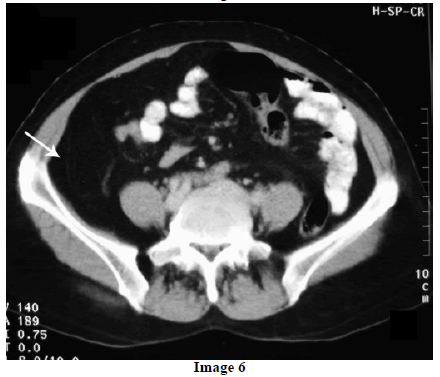
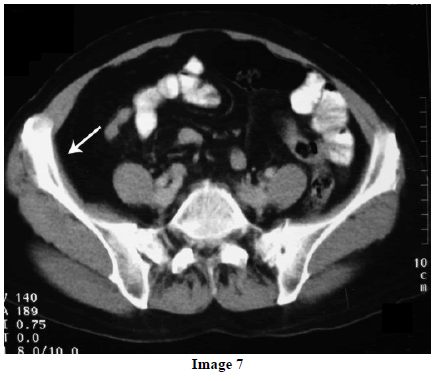
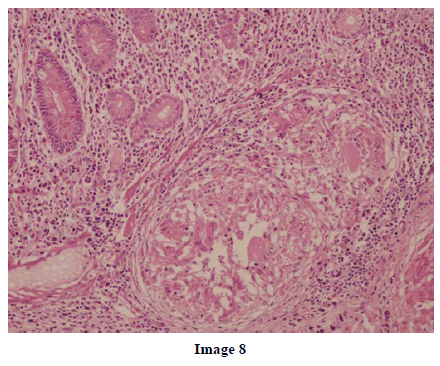
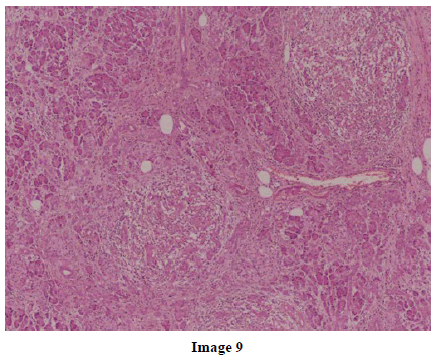
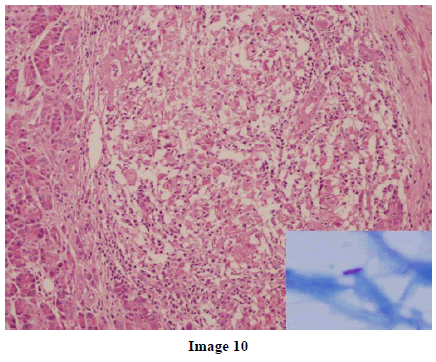
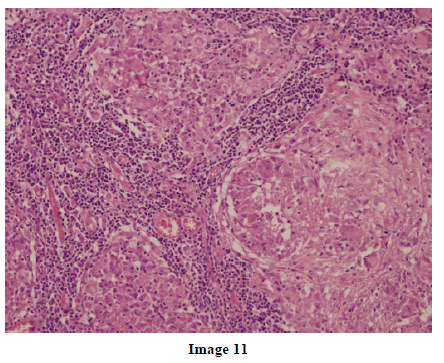
A 45-year-old female presented at our hospital with a history of upper abdominal pain and occasional bilious vomiting for the past two years. There was no history of exposure to tuberculosis. On palpation, epigastric tenderness was present. Routine laboratory investigations were within normal limits. Liver function tests showed: bilirubin, 1.1 g/dL (reference range: 0-1.1 g/dL); alkaline phosphatase, 50 IU/mL (reference range: 30-85 IU/mL); AST, 136 IU/L (reference range: 8-46 IU/L) and ALT, 107 IU/L (reference range: 0-50 IU/L). Serology for hepatitis and HIV was negative. CA 19.9 was less than 5 U/mL (reference range: 0-70 U/mL). An Ultrasound of the abdomen revealed a mass lesion in the pancreatic head and uncinate process with dilation of the main pancreatic duct. A CECT scan showed an ill-defined heterogeneous mass lesion exhibiting heterogeneous post contrast enhancement in the head and uncinate process of the pancreas. The common bile duct and main pancreatic duct were dilated showing the “double duct sign”. Serial CECT scans showed a large growth arising in the pancreas and involving the duodenum. The growth was encasing the portal vein (Images 1, 2, 3, 4, 5). The body and tail of the pancreas were normal. No lymphadenopathy was seen. The Liver was normal in size, shape and echotexture. No intrahepatic biliary radicle dilation was seen. The gallbladder, spleen and kidneys were normal. Serial CECT scans showed minimal ascites in the paracolic gutters (Images 6, 7). An endoscopic biopsy from the ulceroinfiltrative growth in the ampulla did not reveal any pathological signs. On clinical suspicion of a carcinoma in the head of the pancreas, a Whipple’s pancreaticoduodenectomy was performed. Intraoperatively, there was a 3x3 cm bulky mass in the pancreatic head and uncinate process. Frozen section from a single, small lymph node identified (interaortocaval) suggested a granulomatous lesion. The postoperative course of the patient was unremarkable. The operative specimen received was a Whipple’s pancreaticoduodenectomy specimen the stomach, duodenum and pancreas. At the ampulla, there was an ulceroinfiltrative growth measuring 2x3 cm in diameter. Microscopic examination showed numerous coalescing epithelioid cell granulomas in the pancreas which extended into the duodenal mucosa up to the lamina propria. The adjoining pancreatic tissue showed fibrosis and chronic inflammation. The granulomas were comprised of epithelioid cells, Langhans giant cells and were lined by lymphocytes and fibroblasts. No evidence of malignancy was seen in any of the sections. The pancreatic resection margins also showed granulomatous inflammation. Acid fast bacilli were seen in the Ziehl-Neelson stain. All six lymph nodes isolated showed epithelioid cell granulomas. Epithelioid cell granulomas were seen infiltrating the duodenal mucosa (Image 8; H&E, x200); the duodenal gland can be seen in the upper left corner. Epithelioid cell granulomas with Langhans type giant cells were present throughout the whole substance of the pancreas and infiltrated the duodenal mucosa (Image 9; H&E, x100). High-power magnification shows the epithelioid cell granuloma and pancreatic acini (Image 10; H&E, x200); acid fast bacilli are shown in the inset of Image 10 (Ziehl-Nelson stain, x1,000). The lymph nodes isolated from the specimen also showed confluent epithelioid cell granulomas (Image 11; H&E, x200). The entire growth in the pancreas was sectioned and examined but no malignancy was seen. Anti-tubercular therapy was begun (isoniazid, rifampicin, ethambutol and pyrazinamide). The patient has been followed-up for three months in our GI surgery department. She is on anti-tubercular mediation and taking food orally. There have been no abdominal complaints to date.
We have reported a case of isolated pancreatic tuberculosis which was clinically and radiologically suspected to be a pancreatic carcinoma. Our patient had no history of having had tuberculosis in the past, her chest radiograph was normal, the disease was localized to pancreas, there was no other detectable focus of tuberculosis in the body, and epithelioid cell granulomas and acid fast bacilli were seen in the pancreatic tissue. These findings confirmed that the pancreatic tubercular involvement was primary and did not derive from any other focus in the body. Histologic examination of the pancreatic tissue excluded the presence of a pancreatic carcinoma.
In immunocompetent individuals with miliary tuberculosis, infection of the pancreas by Mycobacterium tuberculosis is rare. In the largest autopsy series of 1,656 tuberculosis patients, fourteen patients had pancreatic tubercular involvement; however, all had widespread miliary tuberculosis. None of the patients had isolated involvement of the pancreas [2]. In a study from India, post mortem analysis of 300 patients with miliary tuberculosis over a period of 12 years did not reveal a single patient with pancreatic involvement. Isolated pancreatic involvement in the absence of miliary tuberculosis is even rarer [3]. When it occurs, it most commonly involves the pancreatic head, followed by the body and the tail [4].

It is hypothesized that the pancreas is protected from tuberculous infection probably because of the pancreatic enzymes which interfere with the seeding of Mycobacterium tuberculosis. Some possible mechanisms of pancreatic infection may be pulmonary disease leading to lymphatic and hematogenous spread to the pancreas, ingestion of infected material from an active pulmonary lesion, reactivation of latent tuberculosis in the pancreatic focus, a toxic-allergic reaction of the pancreas involving an inflammatory response to mycobacterial antigens and direct extension from adjacent organs such as the lymph nodes [4].
A typical pancreatic tuberculosis patient is generally a young female presenting with epigastric pain, fever and weight loss, a history of tuberculosis in the past, residing in area in which there is a high prevalence of active tuberculosis [5]. Night sweats, anorexia, malaise, weakness, obstructive jaundice, gastrointestinal bleeding, secondary diabetes and iron deficiency anemia also occur [6].
In cases of pancreatic tuberculosis, CECT may show a sharply delineated mass in the pancreatic head with irregular margins and peripheral enhancement. However these features are not specific for pancreatic tuberculosis. ERCP shows a normal pancreatogram with stenosis of main pancreatic duct seen rarely [4]. Nagar et al. reviewed 32 patients having pancreatic tuberculosis, of which 14 patients had a history of pulmonary tuberculosis while 18 had primary pancreatic/peripancreatic tuberculosis. Ultrasonography, in their cases, showed a bulky inhomogeneous pancreas in five patients and solitary or multiple hypoechoic collections in 27 patients. Twenty-nine patients showed hypodense collections within the pancreas along with peripancreatic lymphadenopathy on CT scan. The remaining three patients had a complex pancreatic mass lesion [7].
Definitive diagnosis of pancreatic tuberculosis is made by demonstrating caseating granulomas, acid fast bacilli on Ziehl-Nelson staining or the presence of Mycobacterium tuberculosis DNA by a polymerase chain reaction in the tissue obtained from the lesion [5]. Biopsies from the lesion reveal granulomas with or without caseation in over 60% of the cases. Ziehl- Nelson stain is quick and simple but sensitive in only about 50% cases. Cultures are more sensitive (77%) and provide antibiotic sensitivities but can take up to 8 weeks which can delay the diagnosis. PCR allows results to be obtained the same day with up to 77% sensitivities; however, false negative results may occur [5, 8].
Pancreatic tuberculosis can mimic pancreatic carcinoma clinically and radiologically. A review of 58 patients with pancreatic tuberculosis revealed that 35 patients were initially diagnosed as having pancreatic cancer [5]. Therefore, patients should be meticulously investigated to rule out the possibility of a carcinoma and to avoid pancreatic resection surgery. In a case reported by Turan et al., a frozen section of a lymph node was inconclusive and a Whipple’s pancreaticoduodenectomy was performed. Histopathology of the specimen suggested tuberculosis [9].
Most cases of pancreatic tuberculosis respond well to anti-tuberculin therapy as a primary treatment or after surgery, with isoniazid/rifampin/pyrazinamide/ethambutol or streptomycin for 6-12 months [10]. There are no defined criteria for surgical management of pancreatic tuberculosis. Different Authors in the literature have taken different approaches for managing these cases. Some have started anti-tubercular therapy on the basis of a smear/biopsy diagnosis of pancreatic tuberculosis without any surgery [1, 11]; some have performed a laparotomy and when a frozen section gave evidence of tuberculosis, resection was not performed, but instead, anti-tubercular therapy was started [10]; and some have performed Whipple’s pancreaticoduodenectomy and then anti-tubercular therapy was begun [12]. Based on the experience of previous Authors, our own experience, the extreme rarity of primary pancreatic tuberculosis, and the relatively common occurrence of pancreatic carcinoma, we feel that any patient presenting with a pancreatic mass must be assumed to have pancreatic carcinoma until proven otherwise (by physical examination, blood biochemistry, radiology, endoscopic smears or ultrasonogram/CT guided fine needle aspiration cytology or biopsy). The possibility of pancreatic tuberculosis must be kept in mind in cases presenting with pancreatic masses and supporting clinical/biochemical evidence must vigorously be sought. If evidence of tuberculosis is found, antitubercular therapy must be started. Whipple’s pancreaticoduodenectomy is the mainstay of treatment in cases of pancreatic/ ampullary carcinoma however the role of surgery is very limited in cases of pancreatic tuberculosis. We have proposed an algorithm for the treatment and follow-up of patients having a pancreatic mass and clinical suspicion of primary pancreatic tuberculosis (Flow chart).
If regression of the mass does not occur in a patient with clinically confirmed pancreatic tuberculosis even after anti-tubercular therapy, then surgery must be performed and the mass must be resected. Up to now there have been no reports of tuberculosis coexisting with pancreatic carcinoma. In a review of 40 cases of pancreatic tuberculosis presenting as a solitary mass, 18 patients underwent surgery which consisted of exploratory laparotomy (14 cases), pancreaticoduodenectomy (2 cases) and distal pancreatectomy (2 cases). The prognosis of pancreatic tuberculosis is good with only four of the 73 cases reported in literature having died of the disease [12].
To conclude, pancreatic tuberculosis can mimic pancreatic cancer on clinical and radiological examination. If there is the suspicion of tuberculosis in a patient having a pancreatic head mass, the patient must be thoroughly investigated (using laboratory tests and smear/tissue examination) to confirm the existence of pancreatic tuberculosis and to exclude the possibility of pancreatic carcinoma. Each case must be assessed individually for optimum treatment.
Conflict of interest The authors have no potential conflicts of interest
Financial grant None