Research Article - (2023) Volume 8, Issue 2
Providing Suggested Rules for Multiple Primary Cancer Recording, Coding and Registering in Population Based Cancer Registry
Mohammad Hossein Somi1,
Roya Dolatkhah2*,
Iraj Asvadi Kermani2,
Sepideh Sepahi3,
Narges Youzbashi3,
Marzieh Nezamdoust3 and
Behnoush Abedi-Ardekani4
1Department of Liver and Gastrointestinal Diseases, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Hematology and Oncology Research, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Health Information Technology, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Pathology, University of Medical Sciences, Tehran, Iran
*Correspondence:
Roya Dolatkhah, Department of Hematology and Oncology Research, Tabriz University of Medical Sciences, Tabriz,
Iran,
Email:
Received: 16-Nov-2022, Manuscript No. IPJCEP-22-14798;
Editor assigned: 18-Nov-2022, Pre QC No. IPJCEP-22-14798 (PQ);
Reviewed: 02-Dec-2022, QC No. IPJCEP-22-14798;
Revised: 24-Jan-2023, Manuscript No. IPJCEP-22-14798 (R);
Published:
01-Feb-2023, DOI: 10.36648/IPJCEP.23.8.011
Abstract
Objective: Multiple Primary Cancers (MPC) present many coding difficulties, while a distinction should
be made between new cases and those with metastasis and/or extension and recurrence of the
primary ones. We aimed to reflect on the experiences and results of data quality control of the East
Azerbaijan/Iran population-based cancer registry and present our suggested rules for reporting,
recording and registering multiple primary cancers.
Methods: Comparability, validity, timeliness, and completeness of data assessment were performed.
We followed the established “rules for reporting incidence and survival". However, in some cases, we
could not find any related data in published and established rules. As a result, we created an expert
panel as a consulting team including expert oncologists, pathologists, and gastroenterologists to
discuss each case.
Results: Overall, among 21,462 total cancer cases registered in EA-PBCR from 2015 to 2017, 35 cases
were registered as MPCs, as follows: 10 out of 6655 in 2015, 12 out of 7042 in 2016, and 13 out of
7765 in 2017. In most cases, we coded the MPCs as the previous guidelines and provided tables that
were somehow according to guidelines, with some more information and case presentations.
Importantly, these suggested rules included cancers in the blood, breast, lung, stomach, small
intestine, colorectal, bone, prostate, bladder, skin, and some additional hints.
Conclusion: Given the complexity of coding MPCs, we suggested some additional rules for identifying,
recording, coding, and registering multiple primary cancers in the context of the EA-PBCR program.
Keywords
Multiple primary cancers; Comparability; Validity; Timeliness; Completeness; Cancer
registry; Population
Abbreviations
MPC: Multiple Primary Cancer; PBCR: Population Based Cancer Registry; EA-PBCR:
East Azerbaijan Population Based Cancer Registry; IARC: International Agency for Research on Cancer;
SEER: Surveillance Epidemiology and End Results; IACR: International Association of Cancer Registries;
ICD-O: International Classification of Diseases for Oncology; ASIR: Age Standardized Incidence Rate;
NID: National Identification numbers; TNM: Tumor, Node, Metastasis; CT: Computerized Tomography;
MRI: Magnetic Resonance Imaging; BMB: Bone Marrow Biopsy
Introduction
In 2020, there were 19.3 million incident cases of cancer and 10.0 million cancer deaths worldwide. Recent statistics produced by the Iranian ministry of health and medical education indicate that cancer is the third overall leading cause of death in Iran. Given that this mortality has also been rising over the past few decades [1,2].
The value and importance of well-established and high quality Population Based Cancer Registries (PBCR) are un-doubtful in improving and providing epidemiologic cancer researches and health policy making programs [3,4]. Key issues in the evaluation of data quality in PBCRs have four quality indicators including comparability, completeness, validity and timeliness of registry data. "Comparability" indicator is the context in which the coding and classification of international guidelines have been performed, compatible with established and confirmed guidelines. "Completeness" indicator is the context of all included diagnosed incident cancers over a desired period of time in a desired population. "Validity" is the exact proportion of cancer cases registered based on the desired source and characteristics in coding, recording and registering. "Timeliness" refers to timely and rapid cancer data collecting, obtaining and registering over a desired period of time [5]. The rules for registering and recording the multiple primary cancers occurring in the same individual, are among the most challenging dimensions of comparability. Multiple primary neoplasms present many coding difficulties, while a distinction should be made between new cases and those with metastasis and/or extension and the recurrence of the primary ones. Warren and Gates explained Multiple Primary Cancers (MPC) for the first time as "definitive malignant tumors", "distinct tumors", and "excluded metastasis tumors" [6]. Based on International Classification of Diseases for Oncology (ICD-0-3) guidelines, the best and reliable definitions are:
• Two or more separate neoplasms in different topographic sites.
• Certain conditions characterized by multiple tumors.
• Lymphomas which often involve multiple lymph nodes or organs at diagnosis.
• Two or more neoplasms of a different morphology arising in the same site.
• A single neoplasm involving multiple sites whose precise origin cannot be determined.
• Not being the result of metastasis or recurrence of primary cancer.
• The recognition of the existence of two or more primary cancers does not depend on time.
However, as PBCRs have wide implications in the monitoring and updating the International Agency for Research on Cancer/International Association of Cancer Registries (IARC/ IACR) standards and international rules, so different registry programs are comparable in collecting, coding and presenting cancer data worldwide. The last version of ICD-O-3.1 and ICDO- 3.2 has been provided additional international rules for multiple primary cancers including updated table as: "Groups of malignant neoplasms considered to be histologically different for the purpose of defining multiple tumors" and was recommended for use from 2020 by IARC/IACR [7]. At this time, apart from several provided rules for MPCs coding and registering, additional evidences and results should be present for the practical and clinical management and treatment strategies [8]. These evidences will be helpful and applicable for other communities and PBCRs.
The national cancer control program in Iran has remits for the prevention, (early) diagnosis, and treatment of various cancers including the provision of palliative care. PBCR is the key to the success of the national cancer control program in Iran. The most current and reliable data for PBCR in East Azerbaijan has been established to allow accurate estimates of annual statistics in the province and has been presented in 2018, while records from additional sources were also used to improve the completeness and validity of the EA-PBCR [9]. However, PBCRs increase the coverage and quality indicators of cancer registries. These studies have less potential bias compared with pathology and hospital based registries [10].
We undertake this study due to some gaps in the handling of multiple primaries based on current guidelines and to be relevant for many cancer registries in improving their quality of reporting MPCs, especially for newly established population based cancer registries. We aimed to reflect experiences and results of data quality control in EA-PBCR and present our suggested rules and provided rules for reporting, recording and registering the multiple primary cancer cases in cancer registry database.
Materials and Methods
Research Design
The methodology used for the cancer registry in the East
Azerbaijan/Iran Population Based Cancer Registry (EA-PBCR)
was based on the operational program of the national cancer
registry of the ministry of health and medical education
(Figure 1).
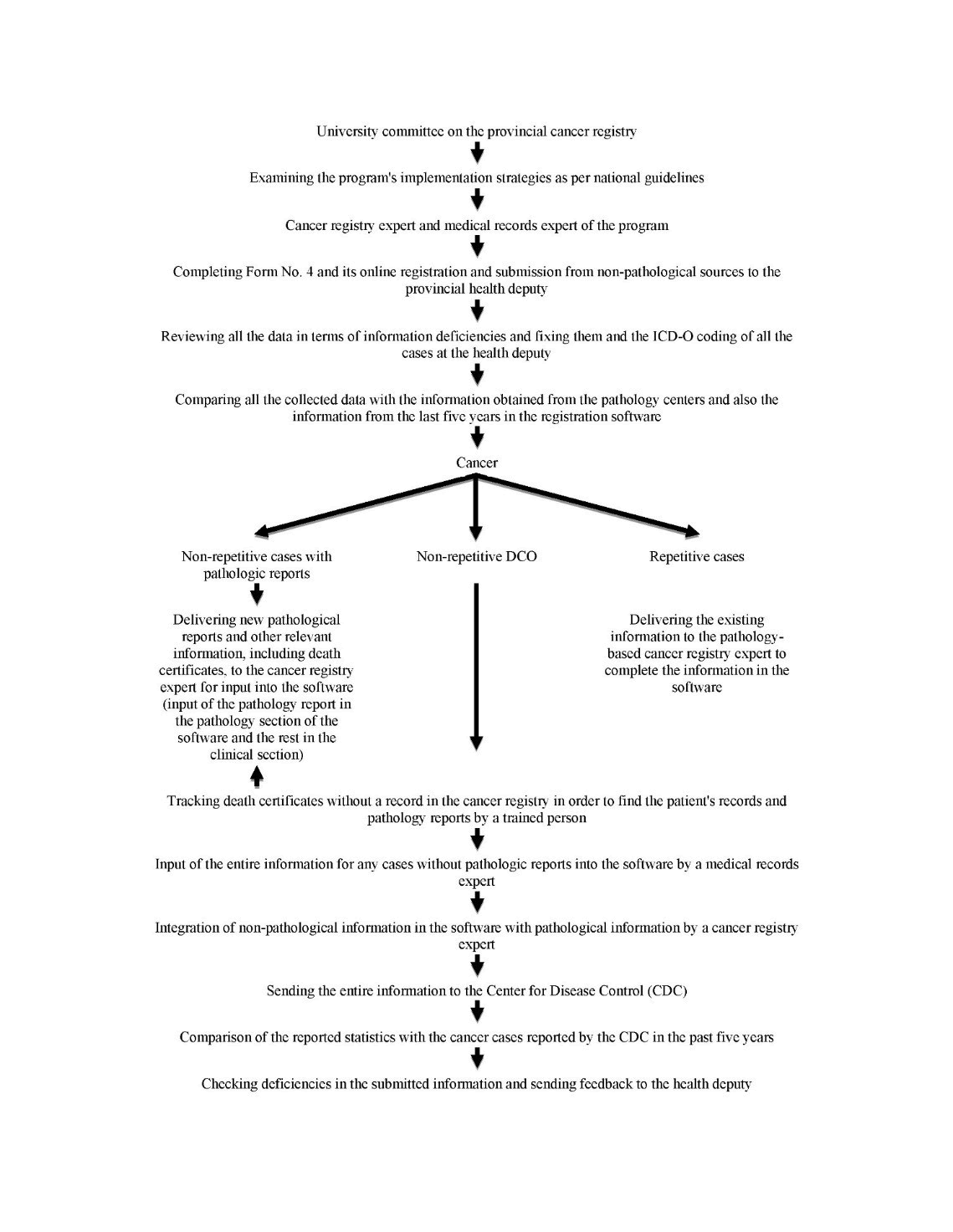
Figure 1: The Population based cancer registry algorithm.
The registered data were all population based and obtained
from the following sources:
• Pathobiology centers.
• Hospital medical records (i.e., The Hospital Information
System: HIS).
• Provincial mortality data.
• Outpatient departments.
• Hematology oncology centers.
• Radiotherapy centers.
• Public and private imaging centers.
• Public and private clinics and health centers.
Data collection was performed using active and passive
methods on a standardized form by well trained staff. Also
some data sources sent the filled information as manually, as
a copy of pathology reports and/or filled standard forms
(paper based).
Study Subjects
East Azerbaijan is the largest and most populous province
located at the Northwest of Iran, while more than 95% of the
local population is of Azeri ethnicity. It covers an area of
45,620 km2 and had a total population of 3,911,278 according
to the 2015 national census in Iran. East Azerbaijan province
includes 20 counties, 62 cities, and 44 districts, with 71.68%
urban composition. The population pyramid for the East
Azerbaijan province by age and sex group, emphasizing that
the population was young, with a predominance of people
aged 20 years-24 years. East Azerbaijan province is in regions
3 of Iran, while the EA-PBCR is held in the capital city, Tabriz.
We included all cases with confirmed primary and newly
diagnosed cancers during 2015 to 2017 years from different
data sources. Data collection was performed using active and
passive methods. During active data collection, the required
cancer registry information was collected from information
sources by the cancer registration personnel from three main
data sources including pathology reports, medical records and
death certificate registry (e.g., we visited hospitals and
collected or copied required data). Additional data collection
was done regularly when the registry office received forms,
copies of discharge summaries and reports from participating
institutions.
After completing data entry, quality control, consistency
checks and basic analysis, manual quality controls and
computerized validity checks of the cancer registry system
were performed based on the IARC criteria in the cancer
registry office of East Azerbaijan province. This involved
assessing factors which influence comparability, validity,
timeliness, and completeness [10-15]. Case duplication across
the registry databases was checked in three steps, first with
patient first name, family name; then by including patient
fathers' name; and finally with patient NID number. Linking
the database with current and previous cancer registries, we
tried to find tumor information from various sources and
improve the validity of our results, and decrease the
percentage of cases obtained from the causes of death
registry without pathology or clinical data were reported as
Death Certificate Only (DCO) cases, and increase the
frequency of cases with microscopic MV or clinical data. The
mortality data were collected from our cancer registry,
observed deaths (reports from follow-up records), or the
national death certificate registry (reported deaths). We then
used data from death certificates and contacted the hospitals
and relatives of patients, which uncovered the clinical or
pathology reports that allowed us to change the basis of the
diagnoses accordingly.
Sample Size Determination
In this study, all newly diagnosed/confirmed cancer cases
(total 21,462) from the year 2015 to 2017 were included for
recording and coding of MPCs.
Measurements
The coding system used for the EA-PBCR is based on the latest
version of the International Classification of Diseases for
Oncology (ICD-O-3). Each tumor is handled as a single entity,
meaning that each patient can have more than one tumor
simultaneously or at different times and that each tumor can
have several reports. We estimated crude incidence rates and
Age Standardized Incidence Rates (ASIR) per 100,000
populations using the 2000 standard world population. Agespecific
incidence rates were also reported for each cancer in
18 strata of 5-years age groups.
Multiple Primary Cancers
The quality control of our database was performed by
computerized and manual validity methods to assess
comparability, validity, timeliness and completeness of EAPBCR
data. Duplicated cases were checked in three steps
using patients' first name, family name, father name and
finally with patients’ National Identification (NID) numbers.
We followed the "Rules for Reporting Incidence and Survival"
published by The International Agency for Research on Cancer
(IARC) and Surveillance Epidemiology and End Results (SEER)
program 19, for multiple primary tumors recording,
identifying, coding and registering; However, in some cases,
we could not find any related data in published and
established rules. As a result, we created an expert panel as a
consultation team including expert oncologists, pathologist,
and gastroenterologist to discuss each case. They discussed
and provided their agreed approach to distinguish each
primary tumor from invasion, metastasis, or recurrence
cancer cases according to the morphology and behavior of
cancers and determined those that are eligible to be coded as multiple primary cases. Meanwhile, MPC in the same organ or
in different organs is a clinical issue; therefore, we referred to
the hospitals and medical records of each case to find any
additional information about the basic information of tumor
progression and TNM staging and/or imaging reports (CT,
MRI).
Results
Meanwhile for the first three years the number and frequency
of registered multiple primary cases are as follow:
• 2015: 10 MPCs out of 6655 cases (4 male and 6 female)
were registered; one had three cancers at the same time,
and 9 had two.
• 2016: 12 MPCs out of 7042 cases (3 male and 9 female)
were registered; all cases had had two primary cancers.
• 2017: 13 MPCs out of 7765 cases (5 male and 8 female)
were registered; one case had three cancers at the same
time, and 12 had two.
We present some additional rules in the context of East
Azerbaijan population-based cancer registry program. In most
cases we coded the MPCs according to the previous
guidelines, and provided tables with some more information
and case presentations. Importantly, these suggested rules for
hematological malignancies are provided for the first time.
The results are summarized and showed in both Tables 1 and
2. All the cases mentioned in the tables were microscopically
verified.
| Label |
Verification (as single primary and/or multiple primary) |
| Blood |
Blood and brain |
In case of definite BMB* results, blood is primary. |
| Blood and bone |
In case of definite BMB* results, blood is primary. |
| Blood and prostate |
Both |
| Blood and stomach |
Both |
| Blood and bladder |
Both |
| Blood and head and neck |
Both |
| Blood and colon |
Both |
| Blood and breast |
Both |
| Breast |
Breast and thyroid |
Both |
| Breast and esophagus |
Both |
| Breast and stomach |
Both |
| Breast and colon |
Both |
| Breast and rectum |
Both |
| Breast and bone |
Breast as primary, bone as metastatic cancer |
| Breast and mediastinum |
Breast as primary, mediastinum as metastatic cancer. |
| |
If thymus was involved we verified both as primary. |
| Breast and liver |
Breast as primary, liver as adenocarcinoma metastatic Cancer. |
| Breast as primary, liver as other morphologies, both. |
| Breast and lung |
Breast as primary, lung as adenocarcinoma, both. |
| Breast as primary, lung as other morphologies, metastatic cancer. |
| Lung |
Lung and head and neck |
Both |
| Lung and sigmoid |
Sigmoid as primary, lung as metastatic cancer |
| Lung and esophagus |
Esophagus as primary, lung as metastatic cancer |
| Lung and stomach |
Stomach as primary, lung as metastatic cancer |
| Lung and skin |
Lung as primary, skin as metastatic cancer |
| Lung and pleura |
Lung as primary, pleura as metastatic cancer |
| Stomach |
Stomach and prostate |
Both |
| Stomach and esophagus |
Both |
| Stomach and ovary |
Both (krukenberg tumor should be rule out) |
| Stomach and colon |
Both |
| Stomach and pleura |
Stomach as primary, pleura as metastatic cancer. |
| Stomach and liver |
Stomach as primary, liver as adenocarcinoma, metastatic cancer. |
| |
Stomach as primary, liver as other morphologies, both. |
| Stomach and small intestine and colon(rectum, recto sigmoid) |
Stomach and colon |
| Small intestine |
Small intestine and Pancreas |
Both |
| Small intestine and peritoneum |
Small intestine as primary, peritoneum as metastatic cancer. |
| Colon and rectum and recto sigmoid |
Colon and cardia |
Both |
| Colon and endometrium |
If both have adenocarcinoma morphology, endometrium is metastatic. |
| If have different morphology, should be registered as multiple primary cancers. |
| Colon and brain |
Colon as primary, brain as metastatic cancer. |
| Colon and liver |
Colon as primary, liver as adenocarcinoma metastatic cancer. |
| |
Colon as primary, liver as other morphologies, both. |
| Colon and prostate |
Both |
| Colon and small intestine |
Both |
| Colon and esophagus |
Both |
| Colon and female genitals |
Both |
| Rectum and female genitals |
Both |
| Sigmoid and ureter |
Both |
| Sigmoid and skin |
Both |
| Recto sigmoid and lung |
Recto sigmoid as primary, lung as metastatic cancer. |
| Recto sigmoid and anus |
Both (FAP and other polyposis should be rule out). |
| Rectum and liver |
Rectum as primary, liver as adenocarcinoma metastatic cancer. |
| |
|
Rectum as primary, liver as other morphologies, both. |
| Bone |
Femur and connective tissue of the upper limb |
Both |
| Femur and other bones |
Both |
| Bone and skin |
Bone as primary, skin as metastatic cancer. |
| Prostate |
Prostate and gallbladder |
Both |
| Prostate and adrenal glands |
Both |
| Bladder |
Bladder and gallbladder |
Both |
| Skin |
Ear Skin and scalp skin |
If both have same morphology, earlier is primary, other one metastatic. |
| Eye Skin and skin of nose and face |
If have different morphology, should be registered as multiple primary cancers. |
| If both have same morphology, earlier is primary, other one metastatic. |
| Skin and parotid gland |
If have different morphology, should be registered as multiple primary cancers. |
| Parotid gland as primary, skin as metastatic cancer. |
| Other |
Esophagus and trachea |
Both |
| Nasopharynx and larynx |
Both |
| Lower limb and soft connective tissue of lower limb |
Both |
| Thyroid and adrenal gland |
Both |
| Liver and brain |
Liver as primary, brain as metastatic cancer |
| Ovary and omentum |
Ovary as primary, omentum as metastatic cancer. |
| Head of pancreas and cerebellum of brain |
Pancreas as primary, cerebellum as metastatic cancer. |
Table 1: Suggested rules for multiple primary cancer recording, coding and registering in East Azerbaijan Population Based Cancer Registry (EA-PBCR), part I
| Label |
Verification |
| Two tumors diagnosed at the same time in colon and rectum |
Primary site should be registered by T stage or tumor sizes. |
| If the above was not available, tumor in third end of distal colon, should be registered as rectum. |
| If tumor was in the other sites of distal colon, should be registered as colon. |
| Two tumors diagnosed at the same time in duodenum and pancreas |
Pancreas is usually invasion of duodenum. |
| Two tumors diagnosed at the same time in colorectal and bladder |
If both have adenocarcinoma morphology, bladder is metastatic. |
| If have different morphology, should be registered as multiple primary cancers. |
| Two tumors diagnosed at the same time in colorectal and liver |
If both have adenocarcinoma morphology, liver is metastatic. |
| If have different morphology, should be registered as multiple primary cancers |
| Two tumors diagnosed at the same time in colon and bladder |
Tumor in sigmoid colon, bladder should be registered as metastasis cancer. |
| Tumor in upper subsides of colon, should be registered as multiple primary cancers. |
| Two tumors diagnosed at the same time in prostate and bladder |
Prostate cancer with adenocarcinoma morphology, bladder should be registered as metastasis cancer. |
| Prostate cancer with transitional morphology, bladder should be registered as primary cancer |
| Two tumors diagnosed at the same time in thyroid and lung |
Thyroid cancer with papillary or follicular morphology, should be registered as primary cancer and multiple primary cancers. |
| Thyroid cancer adenocarcinoma or SCC morphology, should be registered as metastasis of lung. |
| Recto-sigmoid, colon, rectum |
Earlier history |
| Two tumors diagnosed at the same time in pancreas and stomach |
Both invasion should be rule out. |
| Two tumors diagnosed at the same time in liver and pancreas |
Earlier history |
| Female genital |
Earlier history and the rest should be registered as metastasis cancers. |
| Hints |
The rectum can metastasize to the lungs but not to the genital organs. |
| Lymph nodes, omentum, peritoneum, and pleura are mostly metastatic sites. |
| When we have two primary diagnoses with most metastatic possibility, invasion and/or metastasis always should be rule out. |
| When we have two primary diagnoses and metastasis at the same time (patient had a tumor as primary and a metastasis of another tumor with unknown primary site) we keep both. |
Note:
- Recording of MPCs with neuroendocrine components in tumors, collision tumors and other rare tumours (hybrid tumours and others) will need to be classified according to IARC guidelines and coded as in ICD-O to make it comparable and complete.
- MPCs where origin of primary tumour cannot be ruled out, tumors with different morphologies in the same organ, lymphomas and or when determining true second primary is confusing, further consultation with the experts is advisable. Available laboratory reports including IHC can also be helpful.
Table 2: Suggested rules for multiple primary cancer recording, coding and registering in East Azerbaijan Population Based Cancer Registry (EA-PBCR), part I
The identified and recorded multiple primary cancers in our
study during 2015, 2016 and 2017 are provided in details in
(Tables 3-5) respectively.
| |
Age |
Sex |
Topography code |
Morphology |
Final diagnosis |
| Case 1 |
53 |
Female |
C 06.9 |
8070 |
Mouth, squamous cell carcinoma |
| |
|
C 21.1 |
8070 |
Anal canal, squamous cell carcinoma |
| |
|
C 73.9 |
8260 |
Thyroid gland, papillary carcinoma |
| Case 2 |
69 |
Male |
C 16.9 |
8140 |
Stomach, adenocarcinoma |
| |
|
C 18.8 |
8140 |
Overlapping lesion of colon, adenocarcinoma |
| Case 3 |
63 |
Male |
C 16.9 |
8140 |
Stomach, adenocarcinoma |
| |
|
C 19.9 |
8140 |
Recto-sigmoid junction, adenocarcinoma |
| Case 4 |
52 |
Female |
C 19.9 |
8140 |
Recto-sigmoid junction, adenocarcinoma |
| |
|
C 50.9 |
8500 |
Breast, infiltrating duct carcinoma |
| Case 5 |
37 |
Female |
C 26.8 |
8000 |
Overlapping lesion of digestive system adenocarcinoma |
| |
|
C 56.9 |
8140 |
Ovary, adenocarcinoma |
| Case 6 |
66 |
Female |
C 16.9 |
8010 |
Stomach, adenocarcinoma |
| |
|
C 50.9 |
8500 |
Breast, infiltrating duct carcinoma |
| Case 7 |
63 |
Female |
C 50.9 |
8500 |
Breast, infiltrating duct carcinoma |
| |
|
C 67.9 |
8130 |
Bladder, papillary transitional cell carcinoma, non-invasive |
| Case 8 |
84 |
Female |
C 50.9 |
8520 |
Breast, lobular carcinoma |
| |
|
C 73.9 |
8260 |
Thyroid gland, papillary adenocarcinoma |
| Case 9 |
77 |
Male |
C 18.9 |
8140 |
Colon, adenocarcinoma |
| |
|
C 22.0 |
8170 |
Liver, hepatocellular carcinoma |
| Case 10 |
56 |
Male |
C 16.9 |
8140 |
Stomach, adenocarcinoma |
| |
|
C 73.9 |
8140 |
Thyroid gland, adenocarcinoma |
Table 3: Multiple primary cancers reports during 2015, based on our provided rules
| |
Age |
Sex |
Topography code |
Morphology |
Final diagnosis |
| Case 1 |
48 |
Female |
C 44.3 |
9080 |
Skin of face, basal cell carcinoma |
| |
|
C 18.7 |
8140 |
Sigmoid colon, adenocarcinoma |
| Case 2 |
51 |
Male |
C 44.9 |
8090 |
Skin, basal cell carcinoma |
| |
|
C 20.9 |
8140 |
Rectum, adenocarcinoma |
| Case 3 |
57 |
Female |
C 56.9 |
8140 |
Ovary, adenocarcinoma |
| |
|
C 50.9 |
8500 |
Breast, ductal carcinoma |
| Case 4 |
53 |
Female |
C 73.9 |
8260 |
Thyroid gland, papillary carcinoma |
| |
|
C 64.9 |
8312 |
Kidney, renal cell carcinoma |
| Case 5 |
86 |
Male |
C 50.9 |
8500 |
Breast, infiltrating duct carcinoma |
| |
|
C 16.9 |
8144 |
Stomach, adenocarcinoma, intestinal type |
| Case 6 |
56 |
Female |
C 54.1 |
8800 |
Endometrium, sarcoma |
| |
|
C 18.9 |
8140 |
Colon, adenocarcinoma |
| Case 7 |
78 |
Female |
C 56.9 |
8140 |
Ovary, adenocarcinoma |
| |
|
C 20.9 |
8140 |
Rectum, adenocarcinoma |
| Case 8 |
63 |
Female |
C 50.9 |
8500 |
Breast, ductal carcinoma |
| |
|
C 18.9 |
8140 |
Colon, adenocarcinoma |
| Case 9 |
88 |
Male |
C 61.9 |
8140 |
Prostate gland, adenocarcinoma |
| |
|
C 44.1 |
8090 |
Eyelid, basal cell carcinoma |
| Case 10 |
67 |
Female |
C 53.9 |
8140 |
Cervix uteri, adenocarcinoma |
| |
|
C 20.9 |
8140 |
Rectum, adenocarcinoma |
| Case 11 |
78 |
Female |
C 50.9 |
8500 |
Breast, infiltrating duct carcinoma |
| |
|
C 18.9 |
8490 |
Colon, signet ring cell carcinoma |
| Case 12 |
60 |
Female |
C 56.9 |
8441 |
Ovary, serous cystadenocarcinoma |
| |
|
C 18.9 |
8140 |
Colon, adenocarcinoma |
Table 4: Multiple primary cancers reports during 2016, based on our provided rules.
| |
Age |
Sex |
Topography code |
Morphology |
Final diagnosis |
| Case 1 |
45 |
Female |
C 18.1 |
8240 |
Appendix, carcinoid tumor |
| |
|
C 55.9 |
8140 |
Uterus, adenocarcinoma |
| |
|
C 16.9 |
8140 |
Stomach, adenocarcinoma |
| Case 2 |
77 |
Male |
C 61.9 |
8140 |
Prostate gland, adenocarcinoma |
| |
|
C 18.7 |
8140 |
Sigmoid colon, adenocarcinoma |
| Case 3 |
64 |
Male |
C 16.0 |
8145 |
Cardia, carcinoma, diffuse type |
| |
|
C 67.9 |
8130 |
Bladder, papillary transitional cell carcinoma |
| Case 4 |
74 |
Female |
C 23.9 |
8140 |
Gallbladder, adenocarcinoma |
| |
|
C 67.9 |
8130 |
Bladder, papillary transitional cell carcinoma, non-invasive |
| Case 5 |
68 |
Male |
C 18.0 |
8140 |
Cecum, adenocarcinoma |
| |
|
C 64.9 |
8260 |
kidney, papillary adenocarcinoma |
| Case 6 |
52 |
Female |
C 16.9 |
8145 |
Stomach, carcinoma, diffuse type |
| |
|
C 17.1 |
8140 |
Jejunum, adenocarcinoma |
| Case 7 |
59 |
Female |
C 50.9 |
8500 |
Breast, infiltrating ductal carcinoma |
| |
|
C 42.1 |
9835 |
Bone marrow, precursor cell lymphoblastic leukemia |
| Case 8 |
70 |
Female |
C 16.9 |
8144 |
Stomach, adenocarcinoma, intestinal type |
| |
|
C 54.1 |
8380 |
Endometrium, endometrioid carcinoma |
| Case 9 |
51 |
Male |
C 44.3 |
8090 |
Skin of face, basal cell carcinoma |
| |
|
C 71.9 |
9400 |
Brain, astrocytoma |
| Case 10 |
77 |
Male |
C 44.3 |
8090 |
Skin of face, basal cell carcinoma |
| |
|
C 18.7 |
8140 |
Sigmoid colon, adenocarcinoma |
| Case 11 |
50 |
Female |
C 56.9 |
8010 |
Ovary, carcinoma |
| |
|
C 20.9 |
8010 |
Rectum, carcinoma |
| Case 12 |
60 |
Female |
C 17.9 |
8140 |
Small intestine, adenocarcinoma |
| |
|
C 56.9 |
8380 |
Ovary, endometrioid carcinoma |
| Case 13 |
36 |
Female |
C 34.9 |
8041 |
Lung, small cell carcinoma |
| |
|
C 73.9 |
8340 |
Thyroid gland, papillary carcinoma, follicular variant |
Table 5: Multiple primary cancers reports during 2017, based on our provided rules
Discussion
The rules suggested in this study for multiple primary cancers
were provided from our previous 3 years experiences in
the EA-PBCR for reporting data on cancer incidence. All
newly diagnosed/confirmed cancer cases (total 21,462)
from the year 2015 to 2017 were included for recording and
coding of MPCs. In most cases, we coded the MPCs as the
previous guidelines, however, additional suggested rules
provided for malignancies of the blood, breast, lung,
stomach, small intestine, colorectal, bone, prostate, bladder,
skin, and some additional hints. All the cases mentioned were
microscopically verified. The provided hints emphasized
that in multiple primary diagnosis, invasion and/or
metastasis always should be rule out, and some organs
including lymph nodes, omentum, peritoneum, and
pleura are mostly metastatic sites. However the
morphological aspects of multiple cancers always may help
us to differential diagnosis of the MPCs. Recording, coding
and studying of multiple primary cancers provide an insight
into gene-environmental carcinogenesis in individuals with
different cancers at different times. Evaluating and
following of these cases can lead to risk estimation and
familial aggregation, studies of potential familial cancers
and risk of developing second or third cancers.
Meanwhile, detecting and recording of these cases may
provide some information about chemical and radiation side
effects of different cancer treatment protocols which lead
to an increased risk of another cancer and also the
quality of cancer care they received. Furthermore, diagnoses
of multiple primary cancers in early stages and even
asymptomatic cases have the same value along with all other cancer surveillance efforts. Nowadays, two guidelines are
more widely used worldwide for multiple primary cancers,
Surveillance Epidemiology and End Results (SEER) program,
and International Association of Cancer Registries (IACR) and
the IARC rules [15-19]. Based on IARC/IACR
recommendations, all established rules for MPCs are
comparable among different populations. Each PBCR may
provide and suggest some specific and more detailed rules,
and also share with other registries. These data together may
be used to conform to the international rules for PBCR data
quality indicators. Warren and Gates emphasized for the first
time that the incidence of multiple primary cancers is more
than to be counted as accidental. Also they provide some
more information about synchronous and/or metachronous
nature of second primary cancer based on the time of tumor
onset. By improving treatment outcomes of cancer patients,
because of early diagnosis and screening modalities, and also
specific and targeted chemo-radio-therapy protocols, the
number of MPCs increased in the last decades. However, it
has recently been approved that there was inheriting
susceptibility of familial multiple primary cancers in different
populations. It has been revealed that a second or more
primary cancer risk is higher in cancer survivors than normal
population, and the time interval between first and other
primary cancers differed, but is high after approximately 10
years for all cancers and all sites. This is because of genetic
susceptibility in familial cases and side effects of different
cancer treatment modalities. All cancer survivors should
benefit and are mandated to continue the screening and early
diagnosis of cancer at any ages and conditions for any MPCs.
As the aging of the populations and increasing the number of cancer survivors the occurrence and incidence of MPCs is
likely to increase. The incidence of MPCs varies from 2.15% to
17.2% in USA, and 2.4 to 8.17% in different European
countries, however geographical region, coverage of cancer
registries, mean follow-up times (for metachronous MPCs)
and definition used for recording MPCs had significant impact
on this percentages. As the low coverage of population based
cancer registry in most Asian countries, this percentages are
lower than most developed countries. The percentage of
MPCs in our study is 0.16% (35 out of 21,462 registered
cases), which is comparable with previous years and
neighboring countries. The collaborative studies are
important to share any new rules and experiences between
PBCRs, which provide information and evidence about the
potential causes and absolute risks of different types of
multiple primary cancers and their estimated risks. Local and
international agreement for recording, coding and
classification of multiple primary cancers are important in any
new established PBCR program. Recently, by availability of
new chemo and radio-therapy protocols and with increasing
survivorship from different primary cancers, we face with an
increasing risk of secondary cancers. Therefore, establishing
and using comprehensive and principal rules for secondary
cancers as "true second primary cancer" are necessary in
every local PBCR. However, the IARC and IACR provide some
useful international as well as conservative and general
guidelines. Iran has faced an increase in the incidence of all
cancers by about 10% over the last 10 years, but this has
occurred in tandem with a declining trend in the mortality
rates by about 10% over the same period. The EA-PBCR has
helped us to provide important information about the high
incidence of gastrointestinal cancers in this province, (notably
gastric and colorectal cancers), and non-gastrointestinal
cancers (such as breast, Lung, and thyroid cancers).
Prevention and early diagnosis strategies, particularly those of
the gastrointestinal tract, breast and lung cancers must now
be considered as public health priorities both at national and
local levels [20].
Conclusion
We wanted to present some additional rules for identifying,
recording, coding and registering multiple primary cancers in
the context of East Azerbaijan population-based cancer
registry program. The quality of the EA-PBCR is promising
however the study design was not in a systematic approach
but to provide some ideas and "providing suggested rules for
multiple primary cancer recording, coding and registering" in
our cancer registry and provide of some of the available data
on multiple primaries.
Strengths and Limitations
• This study design was not in a systematic approach but
to provide some ideas and "Providing suggested rules for
multiple primary cancer recording, coding and
registering". As of our knowledge, these suggested rules
for hematological malignancies were provided for the
first time.
• Data collection and quality and coverage of data sources
still remain as our major limitations.
• We could not analyze the inherited and cancerpredisposing
mutations in MPC cases. This information
will be helpful to evaluate even early diagnosis of cancer
risk in second or more other sites, and risk estimation in
first and/or second-degree relatives of cases.
• Due to time limitation of the study (3 years) we
presented here only newly diagnosed cancers during the
2015 to 2017 years, as synchronous MPCs, and we are
working on our next studies on most comprehensive and
mono-chromos cases, as including wider follow-up
times.
• Improving data collection quality and adding additional
information including molecular and genetic diagnosis of
the cancers will be our uppermost aim in our upcoming
reports.
Ethics Approval and Consent to Participate
This study has been approved by the ethics committee of
Tabriz university of medical sciences as a confirmed research
project (Code: IR.TBZMED.REC.1396.524). As the ethics rules
of EA-PBCR, all patients’ information and records are
confidential.
Consent for Publication
As the ethics rules of EA-PBCR, all patients’ information and
records are confidential. Written informed consent to publish
this information was obtained from study participants.
Availability of Data and Materials
Data are openly available in a public repository that issues
datasets with the responsibility of the corresponding author.
Competing Interests
The author reports no conflicts of interest in this work.
Funding
This study was supported and funded based on population
based cancer registry program of East Azerbaijan from
ministry of health and medical education, deputy of research
technology and covered the design of the study and
collection, analysis, and interpretation of data (Grant
Number: 700/1480, 1395.10.4). This study has been approved
by the ethics committee of Tabriz university of medical
sciences as a confirmed research project (Code:
IR.TBZMED.REC.1396.524).
Author's Contributions
RD, SS, NY, and MN analysed and interpreted the patient data
regarding the guidelines. MHS, IAK, and BAA performed the
quality control of the data set, and were major contributors in
writing the manuscript. All authors read and approved the
final manuscript.
Acknowledgements
The datasets analyzed and presented in this study are
available from the corresponding authors on reasonable
request.
Disclaimer
Where authors are identified as personnel of the international
agency for research on cancer/world health organization, the
authors alone are responsible for the views expressed in this
article and they do not necessarily represent the decisions,
policy or views of the international agency for research on
cancer/world health organization.
References
- Sung H, Ferlay J, Siegel RL (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 194(7):607-618.
[Crossref] [Google Scholar] [PubMed]
- Hajizadeh A, Monaghesh E (2021) Telehealth services support community during the COVID-19 outbreak in Iran: Activities of ministry of health and medical education. Inform Med Unlocked. 24:100567.
[Crossref] [Google Scholar] [PubMed]
- Bray F, Parkin DM (2009) Evaluation of data quality in the cancer registry: Principles and methods. Part I: Comparability, validity and timeliness. Eur J Cancer 45(5):747-755.
[Crossref] [Google Scholar] [PubMed]
- Parkin DM, Bray F (2009) Evaluation of data quality in the cancer registry: Principles and methods Part II. Completeness. Eur J Cancer. 45(5):756-764.
[Crossref] [Google Scholar] [PubMed]
- Warren S, Ehrenreich T (1944) Multiple primary malignant tumors and susceptibility to cancer. Can Res. 4(1):554-570.
[Google Scholar]
- Vogt A, Schmid S, Heinimann K (2017) Multiple primary tumours: Challenges and approaches, a review. ESMO Open. 2(2):e000172.
[Crossref] [Google Scholar] [PubMed]
- Somi MH, Dolatkhah R, Sepahi S (2018) Cancer incidence in the East Azerbaijan province of Iran in 2015–2016: Results of a population based cancer registry. BMC Public Health. 18(1):1266.
[Crossref] [Google Scholar] [PubMed]
- Wanner M, Matthes KL, Korol D, Dehler S, Rohrmann S (2018) Indicators of data quality at the cancer registry Zurich and Zug in Switzerland. Biomed Res Int. 18:7656197.
[Crossref] [Google Scholar] [PubMed]
- Arndt V, Holleczek B, Kajuter H (2020) Data from Population based cancer registration for secondary data analysis: Methodological challenges and perspectives. Gesundheitswesen. 82(1):62-71.
[Crossref] [Google Scholar] [PubMed]
- Sanchez DR, Barranco MR, Ameijide A (2021) Cancer incidence estimation from mortality data: A validation study within a population based cancer registry. Popul Health Metr. 19(1):18.
[Crossref] [Google Scholar] [PubMed]
- Subedi R, Dhimal M, Budukh A, Gyawali P, Jha AK (2020) challenges and way forward for establishing population based cancer registry in Nepal. J Nepal Health Res Counc. 18(3):544-546.
[Crossref] [Google Scholar] [PubMed]
- Wei W, Zeng H, Zheng R (2020) Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 21(7):342-349.
[Crossref] [Google Scholar] [PubMed]
- Bray F, Ferlay J, Laversanne M (2015) Cancer incidence in five continents: Inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cance. 137(9):2060-2071.
[Crossref] [Google Scholar] [PubMed]
- Ditsch N, Untch M, Thill M (2019) AGO recommendations for the diagnosis and treatment of patients with early breast cancer: Update 2019. Breast Care (Basel). 14(4):224-245.
[Crossref] [Google Scholar] [PubMed]
- Goldschmidt CD, Vathaire FD (2019) Review of risk factors of secondary cancers among cancer survivors. Br J Radiol. 92(1093):20180390.
[Crossref] [Google Scholar] [PubMed]
- Amikura K, Ehara K, Kawashima Y (2020) The risk of developing multiple primary cancers among long term survivors five years or more after stomach carcinoma resection. Tohoku J Exp Med. 250(1):31-41.
[Crossref] [Google Scholar] [PubMed]
- Feller A, Matthes KL, Bordoni A (2020) The relative risk of second primary cancers in Switzerland: A population based retrospective cohort study. BMC Cancer. 20(1):51.
[Crossref] [Google Scholar] [PubMed]
- Ye Y, Neil AL, Wills KE, Venn AJ (2016) Temporal trends in the risk of developing multiple primary cancers: A systematic review. BMC Cancer. 16(1):849.
[Crossref] [Google Scholar] [PubMed]
- Odani S, Tabuchi T, Nakata K (2022) Incidence and relative risk of metachronous second primary cancers for 16 cancer sites, Osaka, Japan, 2000-2015: Population based analysis. Cancer Med. 11(2):507-519.
[Crossref] [Google Scholar] [PubMed]
- Tanjak P, Suktitipat B, Vorasan N (2021) Risks and cancer associations of metachronous and synchronous multiple primary cancers: A 25 yearS retrospective study. BMC Cancer. 21(1):1045.
[Crossref] [Google Scholar] [PubMed]
Citation: Dolatkhah R, Somi MH, Kermani IA, Sepahi S, Youzbashi N, et al. (2023) Providing Suggested Rules for Multiple
Primary Cancer Recording, Coding and Registering in Population-Based Cancer Registry. J Cancer Epidemiol Prev. 8:011.
Copyright: © 2023 Dolatkhah R, et al. This is an open-access article distributed under the terms of the Creative
Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided
the original author and source are credited.