Keywords
Diosmectite; Adsorption; Heavy metals; Detox; Boswellia serrata; Commiphora molmol
Introduction
The human body is constantly exposed to toxins such as
flavor enhancers, food colorings, pesticides, preservatives
and heavy metals that accumulate over time, giving rise to
health problems. Among the physical methods used to control
toxin absorption, smectite-containing products are widely
recommended. Dioctahedral smectite is natural adsorbent clay
formed from aluminomagnesium silicate. It is not absorbed
from the gastrointestinal tract; it does not cause any systemic
side-effects and is classified as a safe over-the-counter drug.
Diosmectite has been effectively used in the treatment of
several gastrointestinal diseases, including infectious diarrhea
and food allergy [1]. In acute diarrhea in children, this effect was
manifested by a reduction in the duration and frequency of liquid
stools as well as in the number of cases of prolonged diarrhea.
The mechanism of action of diosmectite has been proposed to
involve adsorption of viruses, bacteria, and bacterial toxins as
well as modification of gastrointestinal mucus [2]. The adsorptive
ability of diosmectite is explained by its multilayer organization
and swelling ability, creating a large surface for the exchange
of molecules. In addition, the diffuse negative charges confer a large adsorption capacity for mineral and organic cations [3].
Diosmectite treatment may also repair mucosal integrity, as
suggested by the normalization of the urinary lactulose/mannitol
ratio found in children with acute diarrhea [1]. Recently it was
demonstrated that diosmectite had anti-inflammatory activity
when administered as a post-treatment. Possible mechanisms
include adsorption of luminal antigens, increased of colonic mucin
levels, and potentially a direct modulatory action of cytokine
production by mucosal cells [4]. Whereas diosmectite acts mostly
at the physical level, several other drugs regulate gastrointestinal
balance in other ways. Especially interesting are drugs based
on natural products; this include, among others, Boswellia serrata and Commiphora molmol resins. Some components of
boswellia, including β-boswellic acid, have been suggested as
anti-inflammatory agents, as they act to inhibit serine protease
cathepsin G and microsomal prostaglandin E synthase [5]. C. molmol can protect gastric mucosa against ulcers and other
damage. The protective effect of C. molmol is attributed to its effect on mucus production; it has also been shown to increase
nucleic acid and non-protein sulfhydryl concentration, possibly
mediated by its free radical-scavenging, thyroid-stimulating and
prostaglandin-inducing properties [6]. It has been demonstrated,
that the combination of smectite with other drugs can improve
their efficiency [7]. However, when using combined products, the
interaction of all ingredients must be tested. In the present study
a product containing diosmectite, boswellia and Commiphora resin was tested in terms of its adsorption behavior.
Methods
Determination of adsorption of 3-acetyl-11-
keto-boswellic acid on diosmectite
Boswellia serrata resin was incubated with diosmectite in two
ways. In both experiments the substances were incubated for
3 h at 37°C in PBS buffer (pH=6.5). In the first case, the two
substances were incubated together. The amount of Boswellia serrata was 70 mg and that of diosmectite was 70 mg or 3000 mg
(two variants). After incubation, the samples were centrifuged,
dried for 12 h at 60°C and subsequently incubated for 30 min
in 5 mL of methanol. In the second case, both substances were
incubated in a dissolution testing instrument (PT-DT7, PharmaTest
Apparatebau AG, Germany) and were separated by a cellulose
filter (MN 619, Macherey-Nagel). After incubation, diosmectite
was dried for 12 h at 60°C and subsequently incubated for 30 min
in 5 mL of methanol. Detection of 3-acetyl-11-keto-boswellic acid
in the methanol-extract was performed using HPTLC method.
HPTLC analysis of 3-acetyl-11-keto-boswellic acid
The HPTLC analyses were performed on Kieselgel 60 plates
(Merck, Germany) that were eluted in the ascending mode of
a glass chamber with a hexane: acetone: ethyl acetate: acetic
acid (5:2:2:0.5 v/v/v/v) mixture. Samples were spotted on the
TLC plate 10 mm from the bottom edge using a Linomat V semiautomatic
spotter (Camag, Switzerland) and were analyzed using
a TLC Scanner (Camag, Switzerland) at 250 nm. As a reference, we
used 3-acetyl-11-keto-boswellic acid (Sigma- Aldrich, Germany).
Determination of adsorption of histamine on
Symbio®detox
100 mg histamine was incubated for 3 h at 37°C in 50 mL PBS
buffer (pH 6.5) with 4500 mg Symbio®detox. After incubation, the
samples were centrifuged and supernatants were diluted 1:100
in PBS buffer. Histamine in the solution was determined by an
ELISA method using a RIDASCREEN® Histamine Kit (R-Biopharm
AG, Germany), according to the manufacturer’s instructions.
After the substrate reaction, optical density was measured at 450
nm on a plate reader (BioTek Synergy HTX, BioTek® Instruments,
Inc., USA). Concentrations of histamines were calculated using
guidelines from the RIDASCREEN® Kit. Ridasoft Win PC-Software
was used for evaluation of the data.
Determination of adsorption of heavy metals
and NH4+ on Symbio®detox
The metals were incubated for 90 min in 100 mL water solution at
pH 2.0 and pH 6.5 with Symbio®detox, up to 0.5 g of the product. Subsequently, the suspension was centrifuged and the ions were
determined in the supernatant. The concentrations of ions were
0.8 mg/L for Pb2+, 0.09 mg/L for Hg+2, 8 mg/L for Zn+2 and 20 mg/L
for NH+. The determination of mercury (Hg2+) was performed
according to guideline DIN EN ISO 12846 [8] using the cold vapor-
AAS method (MWS DMA 80, MLS GmbH ). The determinations of
Zinc (Zn2+) and lead (Pb ) were performed according to guideline
DIN EN ISO 11885 [9] using inductively coupled plasma optical
emission spectrometry (ICP-OES) (Varian VISTA Pro, Varian Inc.).
The determination of NH4+ was performed according to guideline
DIN 38406-0 [10] using photometric measurements at 655 nm
(LP2W filter photometer, Dr. Lange).
Results
Adsorption of 3-acetyl-11-keto-boswellic acid on
diosmectite
To determine the adsorption of boswellic acids on diosmectite, Boswellia serrata resin was incubated with Symbio®detox in
phosphate buffer at pH=6.8, according to the duodenal flora.
As representative of boswellic acids, we quantified 3-acetyl-11-
keto-boswellic acid. Due to low water solubility, no boswellic
acids could be detected in the solution. Independent of the
incubation method, no adsorption of 3-acetyl-11-keto-boswellic
acid on diosmectite was observed.
Adsorption of histamine on Symbio®detox
91.25% of histamine from the solution was adsorbed on 4500
mg Symbio®detox at pH=6.8. This corresponded to an adsorption
capacity of 0.19 mg/g.
Adsorption of heavy metals and NH4+on
Symbio®detox
The adsorption of ions at pH 2.0 and pH 6.5 on Symbio®detox with
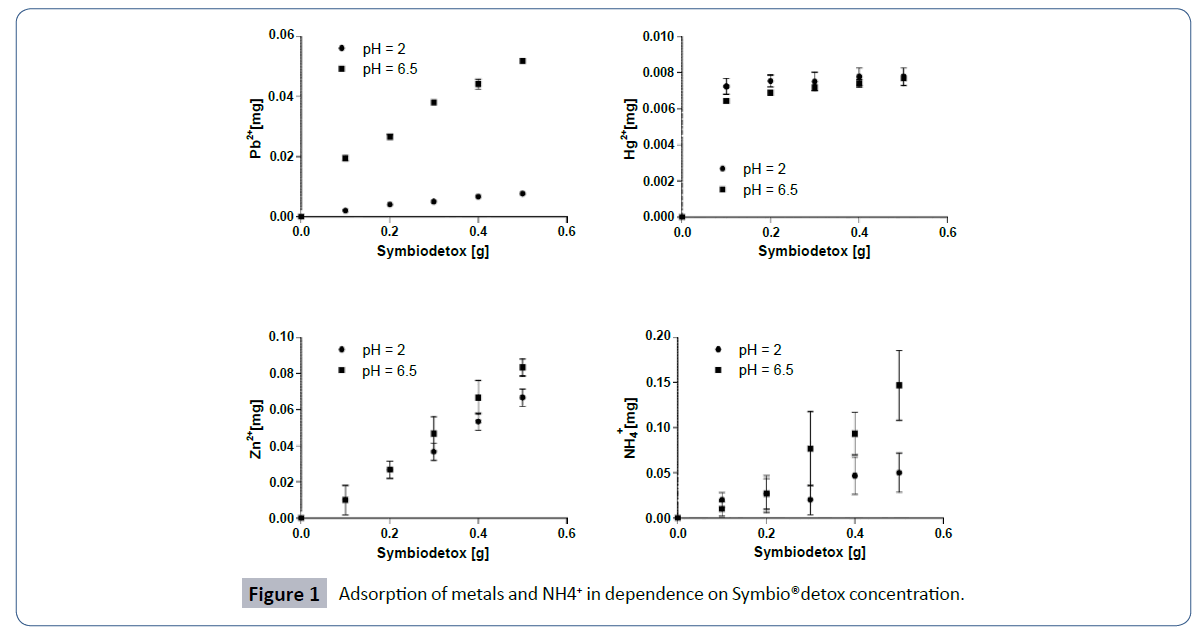
respect to the amount of the adsorbent is presented in Figure 1.
The amounts of adsorbed ions were proportionally dependent on
the amount of Symbio®detox. This behavior was least noticeable
for Hg2+ ions, likely due to a low concentration of the element.
Hence, nearly all Hg2+ ions from the solution remained adsorbed
on 0.1 g of Symbio®detox.
Figure 1: Adsorption of metals Figure 1 and NH4+ in dependence on Symbio®detox concentration.
Adsorption was strongly dependent on the pH of the solution
(Figure 1). At pH=6.5, more Pb2+, Zn2+ and NH+ ions were adsorbed
than at pH 2.0. Only in the case of mercury ions was the opposite
dependence observed. The strongest change in total adsorption
depending on varying pH value was found for Pb2+ ions.
The removal efficiency and the adsorption efficiency for all tested
ions are shown in Table 1. The maximum removal efficiency was
obtained for Hg2+ ions and the lowest removal efficiency was
obtained for NH4 + ions. For solutions with high ion concentration
(Zn2+, NH4
+), the adsorption efficiency was only slightly dependent
on the amount of Symbio®detox. For solutions with low ion
concentration (Hg2+, Pb2+) the adsorption efficiency decreased
with the amount of Symbio®detox. For Hg2+ ions, the adsorption
efficiency decreased from 0.072 ± 0.004 to 0.016 ± 0.001 at pH
2.0 and from 0.064 ± 0.001 to 0.015 ± 0.001 at pH 6.5. For Pb2+ ions, the adsorption efficiency decreased from 0.020 ± 0.001 to 0.015 ± 0.001 at pH 2.0 and from 0.194 ± 0.009 to 0.103 ± 0.001
at pH 6.5. The adsorption capability of heavy metal ions followed
the order Zn2+ >Hg2+ >Pb2+ at pH 2.0 and Zn2+ >Pb2+ >Hg2+ at pH 6.5 (Table 1).
Table 1 Removal efficiency and adsorption efficiency relating to 0.5 g Symbio®detox.
| |
pH |
Removal efficiency (%) |
Adsorption efficiency (mg/g) |
| Pb2+ |
2 |
9.5 ± 0.6 |
0.015 ± 0.001 |
| 6.5 |
60.4 ± 1.1 |
0.103 ± 0.001 |
| Zn2+ |
2 |
8.8 ± 0.6 |
0.133 ± 0.009 |
| 6.5 |
11.2 ± 0.6 |
0.167 ± 0.009 |
| Hg2+ |
2 |
98.7 ± 0.1 |
0.016 ± 0.001 |
| 6.5 |
84.6 ± 1.0 |
0.015 ± 0.001 |
| NH4 |
2 |
2.5 ± 1.1 |
0.100 ± 0.043 |
| 6.5 |
7.2 ± 1.8 |
0.293 ± 0.077 |
Discussion
Dioctahedral smectite is natural adsorbent clay formed from fine
sheets of aluminomagnesium silicate. As with most clay minerals,
it is negatively charged because of the isomorphous substitution
of lesser charged cations in either the octahedral or tetrahedral
lattice positions [11]. The negative surface charge is satisfied by
exchangeable metal cations that are retained by electrostatic
forces in the interlayers or on external surfaces proximal to
the surface charge sites. Water strongly interacts with smectite
surfaces, primarily through hydration of the exchangeable metal
cations. This leads to swelling and subsequent interlayer distance
enhancement [12]. The swelling capability associated with
interlayer extension is very important for the absorption potency of smectite. It allows for trapping of larger molecules, and even
bacteria or viruses. In addition, the diffused negative charges
confer a large adsorption capacity for mineral and organic cations
[13].
Symbio®detox is a product containing smectite as well as
pharmaceutically-active ingredients, including boswellic
acids that regulate balance in the gastrointestinal tract. For
effective action, high adsorption efficiency of toxins (such as
heavy metals or histamine) and no adsorption of the enclosed
pharmaceutically-active ingredients on the smectite is necessary.
In the present work, we showed that organic compounds such
as boswellic acids were not bound to diosmectite. This finding
agrees with those of studies indicating that acidic drugs were not
adsorbed onto diosmectite at pH 5.5-8.0, matching conditions
in the gastrointestinal tract [14]. Thus, it can be expected, that
the pharmaceutical activity of boswellic acids would not be
influenced by the presence of smectite. By contrast, we observed
adsorption of all tested metals and histamine from the solutions
onto Symbio®detox. Adsorption of metal ions onto clay minerals
depends on the charge characteristics of the adsorbent as well
as on the metal properties of ionic charge, ionic radius, and their
hard-soft acid-base characteristics. Adsorption is also affected
by factors such as metal concentration, pH, ionic strength,
type and concentration of competing ions, the liquid-solid ratio
and temperature [15]. Due to varying concentrations of tested
substances, a direct comparison of the adsorption parameters
was not possible. However, the concentrations used for metals
and histamine reflect their content in foodstuffs and thus give
the better overview of adsorption efficiency of Symbio®detox
with regard to its detoxification properties. Mercury and lead
are the most commonly occurring environmental pollutions, and
their negative influence on human health is well known [16,17].
By contrast, zinc is an essential mineral involved in numerous
aspects of cellular metabolism, and thus, zinc deficiency can lead
to serious health problems. The removal efficiency of mercury
and lead in the present experiments at pH 6.5 was very high,
up to 60.4% and 84.6% for Pb2+ and Hg2+, respectively. The
removal of Zn2+ was significantly lower, approximately 11.2%.
The removal efficiency at pH 2.0 was 9.5% for Pb2+ and 98.7%
for Hg2+. The removal of Zn2+ in these conditions was 8.8%.
Adsorption onto the smectite was correlated mostly with the
cation-exchange phenomena. This means that adsorption
was primarily drawn to negatively-charged sites on the clays.
The exchange selectivity gives rise to an order of replacement
determined by the concentration of ions, their valence, and their
degree of hydration and hydrated radius. Specific adsorption
increases with decreasing pKa value [18]; therefore, exchange
selectivity for tested metals should be as follows: Hg2+ >Pb2+ >Zn2+. Additionally, the ion with the greater radius will be more
strongly adsorbed [18]. The ionic radius sequence for tested
metals was Pb2+ >Hg2+ >Zn2+. This dependence was also verified
experimentally by other research group [19,20]. They found, using
different clay materials, better adsorption efficiency for lead than
for zinc. Additionally, the adsorption mechanism was highly pHdependent.
Thus, we observed an increase in removal efficiency
for Pb2+ and Zn2+ with increasing pH of aqueous solutions. Several
reasons may be attributed to the increased adsorption of metal
ions relative to solution pH. The surface of smectite, containing
a large number of active sites, may become positively charged at
very low pH values. Thus, the competition between H+ and metal
ions for available adsorption sites increased because of increased
amounts of H+ in solution. However, as pH increases, these
active surface sites become more negatively charged, enhancing
the adsorption of positively-charged metal ions through an
electrostatic force of attraction [21]. Conversely, increasing
pH has been shown to decrease the solubility of metal ions. At
higher pH, the formation of hydroxyl-metal species and their
precipitation in the surface region was reported [22,23]. The
precipitation of zinc hydroxide occurs predominantly at pH>6.75
[24], whereas this effect for lead is observed at pH>6 [22]. Based
on these data, it can be surmised that at pH 2.0, the adsorption
of the ions was associated only with ion-exchange. At pH 6.5,
the remaining ion exchange was responsible for adsorption of
Zn2+ ions, whereas for Pb2+ the increase of adsorption may be
attributed to the precipitation of lead hydroxyl complexes. By
contrast, maximum adsorption of added Hg2+ occurred at pH 2.0.
At pH 6.5, decreased adsorption was observed. A similar effect
was observed by other research groups [25]. Due to low pKa
values of hydrated mercury (II), the behavior of Hg2+ was different
from that of other metals. Therefore, mercury (II) at low pH is
reduced to mercury (I) and is hydrolyzed on the surface of the
clays, resulting in greater adsorption of Hg2+ in acidic conditions.
Since hydrated mercury (II) ions easily hydrolyze at higher pH,
at pH 6.5 no precipitation of HgO occurs [26]. This results in
decreased Hg2+ adsorption. Based on the data, it can be assumed
that Symbio®detox can efficiently bind toxic heavy metals such as
mercury and lead, while sufficient amount of Zn2+ is left free to be
adsorbed by the gastrointestinal system. Although the ammonia
molecule is a nutrient required for life, excess ammonia may
accumulate in an organism and cause alteration of metabolism or increases in body pH. Elevated levels of ammonia accompany
a number of human diseases, including cirrhosis and acute liver
failure, inborn errors of the urea cycle, and Reye’s syndrome.
Ammonia derives from the metabolism of amino acids, and
especially from gluconeogenic transversion of amino acid into
glucose. The intestine is a major site of ammonia production.
Some 15%-30% of the urea synthesized by the liver is degraded
by bacterial ureases in the gut, with the liberation of ammonia
and carbon dioxide [27]. A second source of ammonia from the
gut is the intestinal mucosa itself. The small intestine produces
a substantial quantity of ammonia that is derived primarily from
the metabolism of glutamine removed from arterial blood [28].
The liver is the most important site of ammonia metabolism,
removing toxic ammonia by urea and glutamine synthesis [29].
However, microorganism overgrowth in the intestinal tract can
produce more ammonia than the body is equipped to deal with,
leading to a compromised immune system [30]. One beneficial
supplement that can combat ammonia excess is clay, which
absorbs toxins from the intestinal tract. In the present work,
we showed that Symbio®detox effectively adsorbed NH+ ions.
At pH 2.0, the relatively modest adsorption of ammonium ions
depended on the quantity of Symbio®detox applied. By contrast,
experiments at pH 6.5 show marked adsorption of ammonium.
More than 7% of the ammonium from the solution could be
removed by 0.5 g of Symbio®detox. One possible explanation is
that the ammonia/ammonium equilibrium (NH3/NH+) at pH 6.5
is shifted in favor of ammonia (NH3) deposited on the surface.
Histamine is a biogenic amine that occurs to various degrees in
many foods [31]. In healthy persons, dietary histamine can be
rapidly detoxified by amine oxidases, whereas persons with
low amine oxidase activity are at risk of histamine toxicity
[32]. Histamine uptake by natural zeolites is one of therapeutic
modalities for reducing histamine content in the intestinal
tract [33]. High absorption capacity could also be shown using
Symbio®detox in the present study. More than 91% of histamine
could be removed from the solution.
Conclusion
The investigations at pH 2.0 (simulated gastric milieu) showed
that the Symbio®detox adsorbed lead (9.5%), mercury (98%) and
zinc (8.8%), while ammonium (2.5%) remained to a great extent
unaffected. At pH 6.5 (simulated duodenal flora), we observed
significantly increased of adsorption of lead (60.4%), ammonium
(7.2%) and histamine (91%). The adsorption of zinc (11.2%)
was only slightly higher at pH 2.0. By contrast, the adsorption
of mercury (84.6%) was slightly lower under these conditions.
The adsorption of toxic heavy metals such as mercury and lead
is preferred over that of zinc. Thus, Symbio®detox may well be
applied as a medical product due to its excellent binding capacity
for toxins including heavy metals, ammonia and histamine.
Simultaneously, deficiency of the essential mineral zinc is not
highly likely. Likewise, adsorption of the pharmaceutically-active
ingredients Boswellia serrata resin and Commiphora molmol resin could not be detected.
Acknowledgement
This work was supported by SymbioGruppe GmbH & Co KG, Auf
den Lüppen 8, Germany.
Declaration of Interest
This research is sponsored by SymbioGruppe GmbH & Co KG.
Hans-Jörg Müller is employed by SymbioPharm GmbH, where the
studied product, Symbio®detox, is manufactured. RSC Pharma LTD & Co.KG received fees for the research. The authors assure
that the methods, results, and data depicted in this paper truly
reflect the procedures used and raw data collected during the
studies. The authors alone are responsible for the content and
writing of this paper.
References
- Dupont C, Moreno JL, Barau E, Bargaoui K, Thiane E, et al. (1992) Effect of diosmectite on intestinal permeability changes in acute diarrhea: a double-blind placebo-controlled trial. J Pediatr Gastroenterol Nutr 14: 413-419.
- Brouillard MY, Rateau JG (1989) Adsorption potency of 2 clays, smectite and kaolin on bacterial enterotoxins. In vitro study in cell culture and in the intestine of newborn mice. Gastroenterol Clin Biol 13: 18-24.
- Suter JL, Boek ES, Sprik M (2008) Adsorption of a Sodium Ion on a Smectite Clay from Constrained Ab Initio Molecular Dynamics Simulations. J Phys Chem C 112: 18832-18839.
- Gonzalez R, de Medina FS, Martinez-Augustin O, Nieto A, Galvez J, et al. (2004) Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br J Pharmacol 141: 951-960.
- Abdel-Tawab M, Werz O, Schubert-Zsilavecz M (2011) Boswellia serrata An Overall Assessment of In Vitro, Preclinical, Pharmacokinetic and Clinical Data. Clin Pharmacokinet 50: 349-369.
- AlHarbi MM, Qureshi S, Raza M, Ahmed MM, Afzal M, et al. (1997) Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol 55: 141-150.
- Kobyliak N, Abenavoli L, Falalyeyeva T, Beregova T (2018) Efficacy of Probiotics and Smectite in Rats with Non-Alcoholic Fatty Liver Disease. Ann Hepatol 17: 153-161.
- Miomir Miljkovic (2012) Influence of Bitumen Emulsion and Reclaimed Asphalt on Mechanical and Pavement Design-related Performance of Asphalt Mixtures. DIN EN ISO 12846. Deutsches Institut fÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ãâür Normung pp: 1-205.
- DIN EN ISO 11885 Water quality - Determination of selected elements by inductively coupled plasma atomic emission spectrometry (ICP- OES) (ISO 11885: 2007); German version EN ISO 11885: 2009
- DIN 38406-5: 1983-10 German standard methods for the examination of water, waste water and sludge; cations (group E); determination of ammonia-nitrogen (E 5)
- Borchardt G (1989) Smectites. In Dixon JB, Weed SB, eds, Minerals in Soil Environments. Soil Sci Soc Am, Inc.
- Minarikova M, Fojtikova V, Vyskocilova E, Sedlacek J, Sikut M, et al. (2017) The capacity and effectiveness of diosmectite and charcoal in trapping the compounds causing the most frequent intoxications in acute medicine: A comparative study. Environ Toxicol Pharmacol 52: 214-220.
- Kraepiel AML, Keller K, Morel FMM (1999) A model for metal adsorption on montmorillonite. J Colloid Interface Sci 210: 43-54.
- Albengres E, Urien S, Tillement JP, Oury P, Decourt S, et al. (1985) Interactions between smectite, a mucus stabilizer, and acidic and basic drugs. Eur J Clin Pharmacol 28: 601-605.
- Ding S, Sun Y, Yang C-n, Xu B-h (2009) Removal of copper from aqueous solutions by bentonites and the factors affecting it. Mining Sci Technol (China) 19: 489-492.
- Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18: 149-175.
- Goyer RA (1990) Lead toxicity: from overt to subclinical to subtle health effects. Environ Health Perspect 86: 177-181.
- Pokras M (2005) Essentials of Medical Geology: Impacts of the Natural Environment on Public Health. Environ Health Perspect 113: A780-A780.
- Jiang K, Sun T-h, Sun L-n, Li H-b (2006) Adsorption characteristics of copper, lead, zinc and cadmium ions by tourmaline. J Environ Sci18: 1221-1225.
- Do NY, Park HI (2011) A Study on Adsorption of Pb, Cu, Zn and Cd Onto Natural Clay. Int J Environ Res 5: 413-424.
- Unuabonah EI, Olu-Owolabi BI, Adebowale KO, Ofomaja AE (2007) Adsorption of lead and cadmium ions from aqueous solutions by tripolyphosphate-impregnated Kaolinite clay. Colloids and Surfaces a-Physicochemical and Engineering Aspects 292: 202-211.
- Griffin RA, Shimp NF (1976) Effect of pH on exchange-adsorption or precipitation of lead from landfill leachates by clay minerals. Environ Sci Technol 10: 1256-1261.
- Farrah H, Pickering WF (1979) pH effects in the adsorption of heavy metal ions by clays. Chemical Geol 25: 317-326.
- Frost RR, Griffin RA (1977) Effect of pH on adsorption of copper, zinc, and cadmium from landfill leachate by clay minerals. J Environ Sci Health. Part A: Environ Sci Eng 12: 139-156.
- Yin Y, Allen HE, Li Y, Huang CP, Sanders PF (1996) Adsorption of Mercury (II) by Soil: Effects of pH, Chloride, and Organic Matter. J Environ Quality 25: 837-844.
- Sajidu SMI, Persson I, Masamba WRL, Henry EMT (2008) Mechanisms of heavy metal sorption on alkaline clays from Tundulu in Malawi as determined by EXAFS. J Hazard Mater 158: 401-409.
- Jones EA, Smallwood RA, Craigie A, Rosenoer VM (1969) The enterohepatic circulation of urea nitrogen. Clin Sci 37: 825-836.
- Weber FL Jr., Veach GL (1979) The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterol 77: 235-240.
- Damink SWMO, Deutz NEP, Dejong CHC, Soeters PB, Jalan R (2002) Interorgan ammonia metabolism in liver failure. Neurochem Int 41: 177-188.
- Nakamura E, Hagen SJ (2002) Role of glutamine and arginase in protection against ammonia- induced cell death in gastric epithelial cells. Am J Physiol Gastrointestinal Liver Physiol 283: G1264-G1275.
- Bodmer S, Imark C, Kneubuhl M (1999) Biogenic amines in foods: Histamine and food processing. Inflamm Res 48: 296-300.
- Maintz L, Novak N (2007) Histamine and histamine intolerance. Am J Clin Nutr 85: 1185-1196.
- Selvam T, Schwieger W, Dathe W (2014) Natural Cuban zeolites for medical use and their histamine binding capacity. Clay Minerals 49: 501-512.