Original Article - (2016) Volume 17, Issue 4
Hipolito Duran, Benedetto Ielpo, Eduardo Diaz, Isabel Fabra, Riccardo Caruso, Luis Malavé, Valentina Ferri, Alessandro De Luca, Maria Elechiguerra, Jan Lammel-Lindemann, AntonioCubillo, Rafael Alvarez, Carlos Plaza, Lina Garcia, Yolanda Quijano and Emilio Vicente
Madrid Norte Sanchinarro San Pablo University Hospital, General Surgery Department, Calle Oña 10, 28050 Madrid, Spain
Received December 23rd, 2015 - Accepted February 23rd, 2016
Introduction Surgery of pancreatic adenocarcinoma with curative intent is the only treatment that offer a long-term survival possibility, with a reported 5-year overall survival rate ranging from 15% to 25%. However, it is only an estimation of long term survival in the majority of reports that could be higher than expected. Our aim is to report the real 5-year overall survival rate based on a large series from a single center and match it with similar reports. Material and methods This is a retrospective analysis of patients with pancreatic adenocarcinoma presenting with 5-year survival rate after the operation performed between 2004 and 2010. We also performed a review of the literature searching for similar series to compare to. Results A total of 128 patients had pancreatic adenocarcinoma resection. Seven patients were lost during the follow up and 4 passed in the early post operative period. The 5-year survival rate of the series is 7.69% (9/117). The analysis of our series and the 8 similar series (388 patients) found in literature shows that some of the well known bad prognostic factors as positive lymph node, poor differentiation grade, R1 resection may be present in these patients. None of long surviving patients was in post operative AJCC stage III and IV: it was the only bad prognostic factor. Conclusions Well known bad prognostic factors can be singled-out in patients with actual 5-year post pancreatectomy survival rates. We realize that the coexistence with some bad prognostic factors should be never taken in account to refute the potential curative surgical treatment except for T4 and/or M1 stage diagnosis.
Pancreatic Neoplasms; Pancreatectomy
SPNs solid pseudopapillary neoplasms
Complete surgical resection followed by adjuvant therapy is, up to now, the best treatment option for localized ductal pancreatic adenocarcinoma (DPAC) [1, 2, 3].
This strategy, performed in experienced centers, reports a general 5-year survival rate of 15-20% (defined as “long term survivors”) (LTS). On the contrary, unresectable pancreatic cancers, shows a fluctuating 5-year survival rate that varies between 1-5% [4].
In literature there are some case series of pancreatic adenocarcinomas with LTS. However, most of them do a patient cohort that last long periods of time, from different centers and with different treatment types, therefore, with many biases [5].
Furthermore, most of these studies series present survival rates estimated using the Kaplan Meier method instead of a real survival rates of 5 year patient follow up [3, 6, 7, 8].
As referred by some authors, [9] it is believed that estimated 5-year survival rate is higher than actual 5-year survival numbers. Given this data, there is still a need to present a series of LTS no estimated by statistics, but with real patient follow up.
The aim of this study is to show the series of LTS patients treated and followed at our center, to compare our findings with other previous published series and, finally, to analyze and correlate these findings with well known bad prognostic factors previously described.
This is a retrospective study in which were included a total of consecutive 152 patients harboring a potentially resectable pancreatic cancer. Patients underwent surgery between March 2004 and October 2010. Only patients with histological or cytological confirmed adenocarcinoma have been definitely included in our analysis. A total of 24 patients with other diagnosis were excluded from the analysis: Pancreatic neuroendocrine tumors (n=19), chronic pancreatitis (n=3), Pancreatic intraepithelial neoplasms (n=1) and lymphoplasmocytic pancreatitis (n=1).
Pretreatment evaluation was standardized and consisted of physical examination, thoraco-abdominal CT scan with vascular reconstruction, FDG-PET scan measuring max standardized uptake value (SUV), pancreatobiliary MRI and endoscopic ultrasound with fine needle biopsy.
Jaundice, if present, is usually resolved preoperatively by biliary metallic stent placement.
Up to 2009 after surgery all patients received adjuvant chemotherapy (gemcitabine alone or in combination with other agents). Radiotherapy was administered only in cases with retroperitoneal involvement. Since January 2010 our institution has been exploring the use of protocol-based neoadjuvant therapy before surgical resection with Gemcitabine plus Nab-paclitaxel [1].
A prospective recording of the following data was performed: main demographic data (age, gender, co-morbidity, ASA stage, neoadjuvancy), main operative data (type of surgery, vascular resection), main post operative outcome (complications, hospital stay, adjuvancy), main pathological data (adenocarcinoma differentiation grade, tumoral size, number of retrieved lymphnodes and number of affected lymphatic nodes, neural invasion, lymphovascular invasion and both section and retropancreatic margin involvement) and finally long term follow up data (Overall survival, Disease free survival, site of recurrence).
Tumoral stage was defined according to the TNM stage of the AJCC [10].
Post operative follow up was performed both by surgery and oncology teams.
All pathological data have been independently reviewed by 2 pathologists. If they weren’t in agreement, a third pathologist was asked to revise the specimen.
Surgeries were performed by 8 surgeons, two of them with high experience in HPB surgery. All procedures have been performed in a similar standard fashion in which a Roux-en-Y digestive tract reconstruction was performed with a duct to mucosa pancreatojejunostomy after duodenopancreatectomy.
We define mortality related to surgery if it occurred up to 60 days from the operation.
We define as long term survival patients alive (with or without recurrence) after 60 months from pancreatic resection date.
Outcome analysis was performed for 128 patients; 7 patients were lost during follow up. A total of 4 patients (3.3%) died during the post operative time and therefore were excluded from this series. The 5-year survival was 7.69% (9/117).
Overall mean survival of the remaining 108 patients who died before completing 60 months after the operation was 17 months.
Main patient demographic characteristics are shown in Table 1.

Jaundice is the more frequent diagnostic preoperative symptom (77.7%), followed by abdominal pain and weight loss (33.3%). All 7 patients with jaundice were successfully treated preoperatively by biliary plastic stent positioning, except one who needed metallic stent (wallflex type) as she underwent neoadjuvant treatment.
Main intra operative and post operative as well as pathological data are shown in Table 2.
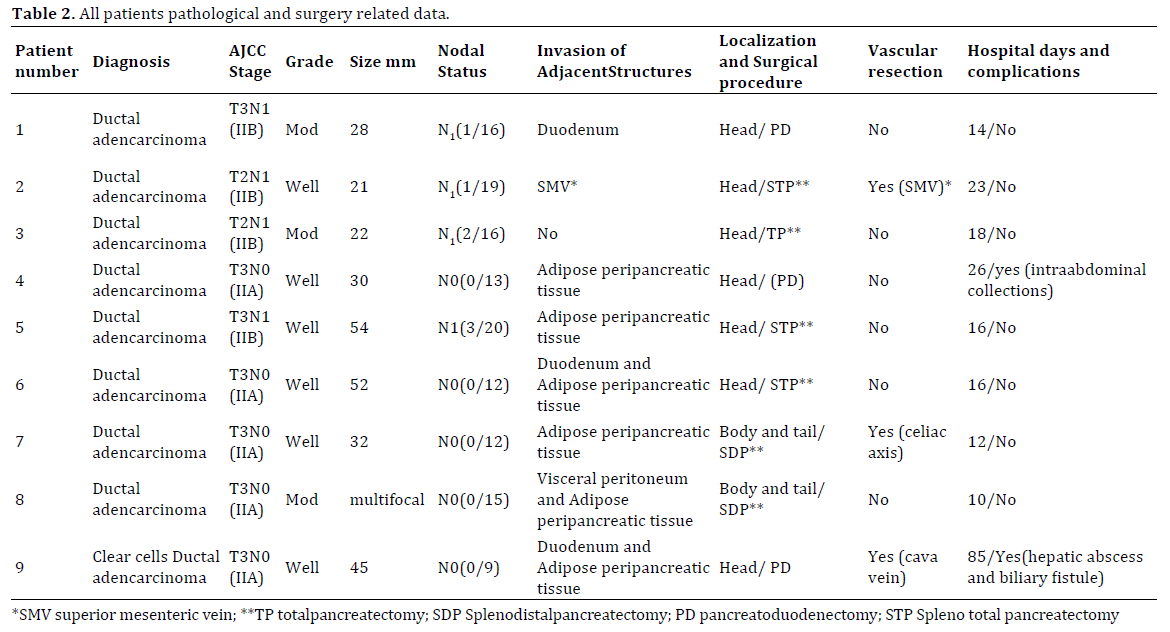
Tumors were located in the pancreatic head in 7 patients, in the pancreatic body in one case and in the last case it was located in the pancreatic tail.
There were 3 concomitant vascular resections (1 case of superior mesenteric vein, 1 case of partially cava resection and one case of celiac trunk resection performing an Appleby technique).
Mean operative time was 370 min. Mean intraoperative blood loss was 300 cc. Mean perioperative blood transfusion was 2.1 units.
A total of 2 patients had post-operative complications. One patient suffered of an intrabdominal abscess treated successfully by radiological drainage; one patient presented left lateral liver ischemia and needed surgical reintervention. Mean hospital stay was 16 days (range: 10-85).
All specimen borders were free from disease (R0). In one case was found retroperitoneal involvement. In all cases, adenocarcinoma was confirmed by pathological and immunohystochemical analysis. One out of the 9 cases showed a clear cell subtype.
Mean tumoral size was 31 mm (range: 21-54 mm). One specimen was found to be a multifocal pancreatic tumor. From the 9 tumors, 6 were well differentiated and the 3 remaining specimens presented moderate differentiation. There was no pathological evidence of tumor involvement in the vascular resection specimens.
Mean number of retrieved lymph nodes was 15 (range: 9-20). In 4 cases (44%) there were positive lymph nodes.
TN staging is shown in Table 2.
Adjuvant treatment and type is shown in Table 3. Adjuvant radiotherapy was administrated only to the patient with retroperitoneal positive border.

The overall mean survival from the time of the operation was 79 months (range: 61-128 months). Today, 4 patients (44.4%) are still alive and without recurrence. One patient that is still alive presented local recurrence (after 90 months from surgery) and has been successfully managed with chemo-radiotherapy. One patient is still alive with pulmonary (presented 50 months after operation) and mediastinal metastases (presented 72 months after operation). A total of 3 patients died secondary to tumoral recurrence at 61, 63 and 73 months after operation, respectively.
Tables 4, 5 summarize our results in comparison with other eight series also collecting actual 5 years survivors after pancreatectomy for DPAC [4, 5, 11, 12, 13, 14, 15, 16].


Most series reporting patient survival after pancreatic resection for DPAC present only estimated survival, calculated by Kaplan-Meier method. However, as referred by some authors, this method may over estimate data for LTS [9].
The aim of this study is to present and analyze the number of LTS after pancreatectomy for DPAC at our center.In literature there are only few similar studies [4, 5, 11, 12, 13, 14, 15, 16]. However, these series, gathered data only up to 2004. As in the last decade there have been important improvement in surgical techniques and adjuvant treatment, there is need to present a newer study showing LTS after DPAC. Our series gathered data from 2004 up to 2010, describing those patients which achieves 5 years of survival.
Our series has incorporated the most important changes that occurred in the past decade treating resectable DPAC. These are the novel targeted drugs (nab-paclitaxel, erlotinib) [1, 2], as well as radiotherapy used before [17] and after operation [18]. As showed in Table 3 patient nº 9 received neoadjuvant chemo-radiotherapy before surgery, patients nº 4, 5 and 7 received new agents as nab-paclitaxel in adjuvant regimen. Finally, in the last decade surgery for local recurrences is also being considered as longer survival terms are achieved after resection. In patients nº 3 and 7 the local relapse was surgically removed.
In the literature it has been reported that histopathology review of LTS can lead to a change in diagnosis in up to 6% of the cases [19]. Because the prognosis for adenocarcinoma of the pancreas is much worse than that of other peri-ampullar and pancreatic tumors, failure to accurately confirm the original pathologic diagnosis undoubtedly results in the inclusion of other tumor types, which can falsely inflate long term survival results. The histopathological specimens of all of our nine patients with a long term survival were re-evaluated by two pathologists specialized on pancreatic diseases. If there was no agreement the specimen was sent to a third one (it was only necessary in patient case nº 9)
Three out of 8 LTS series reviewed in this study, confirm that there has been some specimen misdiagnosis. The Conko-001 [5] study confirms the diagnosis of adenocarcinoma in all but one specimen in which the analysis was repeated and finally corrected to a high grade neuroendocrine carcinoma. Similarly, Katz et al. [12] led to a change in the diagnosis of 3 patients to biliary adenocarcinoma in 2 cases and intraductal papillary mucinous neoplasm in 1 case. Finally, Clearly et al. [15] withdrew one patient from the analysis because pathology revision revealed a diagnosis of duodenal adenocarcinoma.
Given this result, it is possible that other series may have LTS with no DPAC in the specimen, even if they do not address this issue.
The analysis of our series consists of correlate LTS to well known prognostic factors, as follow:
Blood Transfusion
In a number of solid tumors, perioperative blood transfusion has been reported as a negative prognostic factor [20, 21, 22]. An explanation for this finding could be that allogenetic blood transfusion could induce immunosuppression in the host, thus exhibiting a higher recurrence rate in the patient after tumor resection. It has also been advocated that the requirement for blood transfusion correlates with cases that present with advanced tumors and are more difficult to resect.
A meta-analysis conducted by Garcea et al. demonstrates that transfusion of less than 2 U is associated to a longer survival after DPAC operation [23]. Conversely, our nine patients received in the perioperative period a mean of 2.1 blood units, lightly superior.
Among the eight series consulted the only one showing blood loss as an independent factor for LTS was published by Dusch et al. [4].
No Prior Attempt at Resection
Katz et al. [12] include as an adverse factor associated with long-term survival a previous attempt at tumor resection before referral to his institution. Of the 88 5-year survivors, only 5 patients had undergone an unsuccessful attempt at tumor removal before referral. Conversely, 43 patients had undergone laparotomy for planned pancreatectomy before referral in the 241 non 5-year survivors; the effect of an unsuccessful prior laparotomy was profound and elucidates the importance of carefully selecting patients for PDAC surgery who are compatible with the skill set of the surgeon. Some studies and recent guidelines have suggested sending these patients to centers that perform more than 20 cases a year [24, 25]. In any of our nine patients, when referred from other institutions, a prior attempt for resection had been proposed.
R0 Resection
Margin status has been likely associated with the location and size of tumors. Tumors located in proximity to superior mesenteric vessels and celiac trunks are more likely to have specimen margin involvement. However, in the last decade, improvement of the vascular techniques allows for a marked decrease in margin involvement performing vascular resections [26].
In our series of 9 LTS, vascular resection was performed in 3 cases, with pathological vascular involvement in none of them. As reported by some authors, only 50% of resected vessels presented pathological tumoral involvement and only in this case it can be defined as poor prognostic factor [27]. Otherwise, LTS is still possible for these patients, as shown in our series.
In patient nº 2 the superior mesenteric vein was resected and reconstructed. This patient died secondary to the disease 61 months later. In patient nº 7 the celiac axis was resected by an Appelby technique. No vascular reconstruction was needed. Patient is still alive with mediastinum disease 94 months later. In patient nº 9 a segmental inferior vena cava resection was performed. Patient is alive without disease 61 months later.
Is mandatory to insist that vascular resection should not be considered anymore as a factor of poor prognosis unless tumoral pathological involvement of the resected vessel exists [26, 27].
Only in two of the consulted series [12, 14] the R0 margin was predictive of longer survival. Moreover, in 9.87% (36/365) of the 5-year survivors analyzed [4, 5, 12, 13, 14, 15] R1 resections were present. Thus, the presence of margin involvement should never preclude a LTS.
Lymph Node Status (N0)
In four of the listed 5-year actual survival patients the lymph node involvement was associated adversely with long-term survival [5, 12, 13, 16]. Also, lymph node metastases cannot exclude LTS. In fact we found that 44.37% (150/388) of all the 5-year survivors collected in the 8 series consulted had positive lymph nodes at the time of operation. Similarly, in our series 44.44% (4/9) of 5-year survivors had positive lymph nodes.
AJCC Stage
As shown in 6 of the 8 series listed in Table 4 [4, 5, 11, 12, 13, 15] AJCC stage was not a significant prognostic factor for 5-year survival rates. Therefore, even in patients with advanced stages, such as with large tumor size and/ or lymph node metastases, they have a chance for cure through surgical resection. None of the patients in our series were registered as early stages (IA-IB), but all of them were stage II (55.55% IIA; 44.44% IIB). We did not register Stage III-IV patients. Interestingly, none of the patients listed in the series consulted [4, 5, 11, 12, 13, 14, 15, 16], making a total count of 388 patients, were stage III-IV either. The issue is that we cannot understand if the specimen is going to be a pathological T4 stage, unless surgery is performed. Whenever an arterial resection was needed to complete pancreatectomy, pathological involvement of it (pT4) was confirmed only in 50% of cases [27]. Given this data, therefore, arterial resection should be performed in selected cases.
Tumoral Grade
Two studies [5, 15] revealed improved survival rates seen with well differentiated tumors compared with poorly differentiated neoplasms. This implies that the biologic behavior of the tumor affects patient outcomes. Accordingly to this statement, none of our nine patients were poorly differentiated. Moreover, some preclude tumoral grade to be included as an additional factor in the TNM staging system, in order to improve a better selection for patients to be included in further projects testing for new molecular markers [28]. However, we should mention that 32.45% (73/225) of the 5-year survivors analyzed [4, 5, 11, 13, 15, 16] had a contrasting poorly differentiated DPAC.
Adjuvant Chemotherapy
The CONKO-001 trial [5] didn’t only demonstrate the benefit of adjuvant chemotherapy with gemcitabine in DPAC overall survival, but also elucidated this factor as an independent prognostic factor for the 5-year patient survivors. Unfortunately this series has to be interpreted with caution as chemotherapy was routinely given to patients only from 1998 resulting in a limited number of patients having undergone chemotherapy. Another drawback is the absence of standard protocols derived from the vast number of centers enrolled in the study. Similarities are found in the rest of the series consulted, with only a few patients attending a formal chemotherapy regime, as they were treated in previous times when gemcitabine was not prescribed as a standard adjuvant treatment. Conversely, in our series with a recent initial data collecting date, all the patients received neoadjuvant or adjuvant treatment. As mentioned above, every time a disease recurrence was diagnosed, a new chemotherapy treatment regime was tested.
One consideration must be addressed about Katz series [12]. The incidence of patients with neoadjuvant treatment is really high, but is not an independent prognostic factor because very similar figures are present in both groups: 5 year survivors and under 5 year survivors (74% & 78% respectively).
LTS, Recurrence and Death
All of the series consulted registered recurrences and deaths. Most of the recurrences that can occur are local and distant, mainly in lungs, liver and brain [12]. Our series is also consistent with this pattern of late recurrences and deaths. Actual five years, or even ten years survival is not synonymous of cure [14]. The causes of these late recurrences are unclear and different theories have been proposed (occult lymph node or liver metastases, residual low-grade intraductal malignancies, or de novo tumors in the pancreas remanent).
Analysis of the clinical and histopathological variables in the 5-years survival group in the 8 studies selected demonstrates the enormous difficulty in defining prognosis for an individual patient. In a considerable number of these patients we realize the coexistence with really bad prognostic factors that never should be taken in account to refute the potential surgical treatment.
However, its noteworthy to mention that a AJCC stage III-IV is the only bad histopathological prognostic factor that was never present among the 397 patients (the eight 5-years survivors series consulted and ours) listed in this study. We can affirm categorically that a T4 or M1 stage are no longer associated with long term survivors.
According to our experience and the literature review, real LTS rate for patients that underwent pancreatectomy for adenocarcinoma is lower than estimated by KaplanMeier. On the other hand LTS is possible even if some of the well known bad prognostic factors are present.
Acknowledgments
The authors thanks Isabel de Salas, Pablo Ruiz for their contribution
Conflict of Interest
The authors declare no conflict of interest