Research Article - (2023) Volume 8, Issue 2
Recombinant Human Monoclonal Antibodies Casirivimab/Imdevimab use in Inhibition of the Sars-Cov-2 Virus Infection
Monika Beliančinová1,2,
Patrícia Kleinová1,2*,
Karol Graňák1,2,
Matej Vnučák1,2,
Michal Kolorz3,
Marián Mokáň2 and
Ivana Dedinská1,2
1Transplant Centre, University Hospital Martin, Slovakia
2Department of Internal Medicine, University Hospital Martin and Jesseniu, Comenius University, Slovakia
3Department of Pneumology and Phtiseology, University Hospital Martin and Jessenius, Comenius University, Slovakia
*Correspondence:
Patrícia Kleinová,
Transplant Centre, University Hospital Martin,
Slovakia,
Email:
Received: 29-Mar-2023, Manuscript No. IPJHCC-23-16239;
Editor assigned: 31-Mar-2023, Pre QC No. IPJHCC-23-16239 (PQ);
Reviewed: 14-Apr-2023, QC No. IPJHCC-23-16239;
Revised: 19-Apr-2023, Manuscript No. IPJHCC-23-16239 (R);
Published:
26-Apr-2023, DOI: 10.36846/2472-1654-8.2.8018
Abstract
Introduction: The spread of the SARS-CoV-2 virus has caused severe problems for healthcare facilities and infrastructure worldwide. The development of rapid diagnostic tools, effective treatment protocols, and vaccines against the pathogen has accelerated. This work aims to elucidate the benefits of recombinant human monoclonal antibodies to slow the progression of SARS-CoV-2 variant B.1.617.2 infection (delta variant).
Material and methods: This is a retrospective analysis with a 6-month follow-up involving all patients who received recombinant human monoclonal antibodies (MABs) Casirivimab/Imdevimab at University Hospital Martin in November and December of 2021.
Results: A total of 180 patients were enrolled in the cohort with a mean time to administration of symptoms were 6.01 ± 0.3 days in the group of vaccinated patients and 5.52 ± 0.28 days in the group of non-vaccinated patients and a mean time to the resolution of symptoms were 4.37 ± 0.62 days in the group of vaccinated patients and 3.83 ± 0.3 days in the group with non-vaccinated patients. Of these patients, 13 developed bronchopneumonia (7.2%)-serious side effects after MAB administration were observed in 1 patient.
Conclusion: Using recombinant human monoclonal antibodies Casirivimab/Imdevimab to slow or to stop SARSCoV- 2 variant infection B.1.617.2 significantly affected the course of the disease. Quick diagnostics, identification of at-risk patients, and multidisciplinary collaboration are essential in COVID-19 management.
Keywords
SARS-CoV-2; Monoclonal antibodies; Casirivimab/Imdevimab
Introduction
In 2019, the SARS-CoV-2 virus, the causative agent of COVID-19, was identified for the first time. The SARS-CoV-2 virus enters host cells, such as nasal/bronchial epithelial cells or pneumocytes, by binding through its spike protein to the angiotensin- converting enzyme 2 (ACE2) receptor on the cell surface. After the penetration of the virus into the host’s organism, the disease develops in various forms, from an asymptomatic course to the development of a severe course of the disease with multi-organ involvement and respiratory insufficiency, often with the need to use pulmonary ventilation or use of the extracorporeal circulation (ECMO). Recent data show that a more severe course of COVID-19 is associated with a high viral load in the host [1-3].
Thanks to vaccination, we can observe a decline in cases with a moderate to severe course that requires hospitalization. In addition, the administration of the monoclonal antibodies Casirivimab/ Imdevimab, the use of which was first approved by the Food and Drug Administration (FDA) in November 2020 in the United States of America (USA) and later in other countries, was a significant help in preventing the development of a severe course of COVID-19. MABs were approved by FDA for emergency use to treat mild to moderate cases of COVID-19. Recombinant human monoclonal antibodies Casirivimab and Imdevimab combine two gamma one immunoglobulins that act against the spike protein of the SARS-CoV-2 virus, the causative agent of the disease COVID-19.
They show a high affinity for binding to different non-overlapping epitopes of the binding domain of the receptor (VDR) for the spike protein of the SARS-CoV-2 virus [4,5]. Each antibody almost entirely (≥ 95%) blocks in vitro binding of the VDR spike protein of SARS-CoV-2 to the human angiotensin-converting enzyme 2 (ACE2) receptor [5]. Casirivimab and Imdevimab block the binding of the spike protein of the virus to ACE2 already with a half maximum inhibitory concentration of 56.4 pmol/l and 165 pmol/l individually and 81.8 pmol/l in combination [6]. In animal model studies, MABs have shown therapeutic potential when used prophylactically or as a treatment for ongoing COVID-19 infection, limiting airway viral load and virus-induced lung injury in monkeys and body weight loss in hamsters. To date, we know several variants of the SARS-CoV-2 virus, for example, B.1.1.7 (alpha), B.1.351 (beta), P.1 (gamma), B.1.617.2 (delta), and others (Table 1).
| World Health Organization Nomenclature |
| Alpha |
B.1.1.7 |
| Beta |
B.1.351 |
| Gamma |
P.1 |
| Delta |
B.1.617.2 |
| Omicron |
B.1.1.529, BA.2 |
| Epsilon |
B.1.427, B.1.429 |
| Zeta |
P.2 |
| Eta |
B.1.525 |
| Theta |
P.3 |
| Iota |
B.1.526 |
| Kappa |
B.1.617.1 |
| Lambda |
C.37 |
| Mu |
B.1.621 |
Table 1: WHO nomenclature of the SARS-CoV-2 subtypes (6).
Aims
This work aims to elucidate the benefits of recombinant human monoclonal antibodies to slow the progression of SARS-CoV-2 variant B.1.617.2 infection (delta variant) and to determine the benefit of the MABs in the strategy of treating the COVID-19 disease. We determined the average number of days until the disappearance of the symptoms from the administration of MAB, the occurrence of adverse effects including CRS, and the frequency of the consequences of the disease COVID-19 in patients who were submitted to the MAB. Furthermore, the work aimed to determine the proportion of patients vaccinated and required hospitalization for the development of bronchopneumonia associated with COVID-19 compared to patients who were not vaccinated as other comorbidities that increased the rate of hospitalization in patients.
Materials and Methods
MABs Casirivimab/Imdevimab were indicated for slowing or stopping RT-PCR-confirmed COVID-19 in patients aged 12 years and older who are not oxygen dependent (peripheral blood oxygen saturation Ë?90%) and are at high-risk diseases with a severe or very severe course up to death. The infusion time with added MABs was 20 minutes-45 minutes, and the patients were monitored for at least one hour after drug administration. The administration of MAB is associated with the occurrence of adverse reactions, including the occurrence of Cytokine Release Syndrome (CRS).
Therefore, the treatment was given in an outpatient form at the University Hospital in Martin, where it was possible to manage the allergic reaction to the infusion. The infusion was given once and contained 600 mg of Casirivimab and 600 mg of Imdevimab diluted in 250 ml or 500 ml of normal saline solution. Among the risk factors of patients who were indicated for MAB administration were age over 65 years, obesity with BMI over 35 kg/m2, existing cardiovascular disease including arterial hypertension with organ complications, chronic lung disease including asthma and Chronic Obstructive Pulmonary Disease (COPD), diabetes type 1 or 2. Other risk factors include chronic kidney disease, including the need for dialysis, kidney or other solid organ transplant status, hepatic insufficiency, use of immunosuppressive therapy, oncological diseases or chronic infections, and immunodeficiency conditions such as hepatitis or viral infection-Human Immunodeficiency Virus (HIV).
Inclusion criteria for MAB administration and HIV-Human Immunodeficiency Virus are:
• Patients older than 65 years
• Patients with BMI ≥ 35kg/m3
• Patients chronic kidney disease (CKD G3-G5) including hemodialysis patients and patients with nephrotic syndrome
• Patients with chronic hepatic disease in the stage of fibrosis or cirrhosis with hepatic insufficiency signs
• Patients with cardiovascular disease with heart failure or cardiac decompensation in the past
• Patients with arterial hypertension with organ complications
• Patients with chronic pulmonary diseases and chronic respiratory insufficiency or with exacerbations requiring hospitalization in the past
• Patients with diabetes mellitus type 1 or 2 with complications
• Patients with the severe form of Parkinson’s disease or with other neurological diseases with the risk of respiratory failure during COVID-19. Patients with severe cases of immunodeficiency or Down syndrome with obesity or other severe complication associated with Down syndrome
• Patients with immunodeficiency
• Patients with hemato-oncological therapy after organ transplantation, or after transplantation of the hematopoietic cells
• Patients with badly controlled HIV infection or on immunosuppressant’s
Statistical Analysis of Data
We evaluated all data using descriptive statistics, with data normality (normality of data distribution) assessed using the Shapiro- Wilk test. In addition, we evaluated differences between groups using the Student’s T-test for parametric testing and the Mann-Whitney U-test for non-parametric group testing. To assess the correlation between groups, we chose the Pearson correlation coefficient. A value of p<0.05 and 0.001 in the analysis was considered statistically significant. For statistical data processing, we used commonly available statistical tools on the Internet and the MDCALC program, version 20.2.15.
Results
A total of 180 patients were enrolled in the cohort, of which
82 were vaccinated, and 98 patients did not receive any of the
SARS-CoV-2 vaccines before MAB administration. The groups
were made up of 61.76% women and 38.24% men. A total
of 60 patients (33.3%) used antibiotics along with supportive
therapy, of which eight patients using ATB developed bronchopneumonia
(BRPN), and three were hospitalized (p=0.32). In
5 patients with BRPN, treatment did not include added ATB.
Bronchopneumonia occurred in a total of 13 patients (7.2%),
of which four patients were vaccinated, nine patients were
not vaccinated with any vaccine against SARS-CoV-2 (p=0.13),
of which three were vaccinated (3.7%). 6 unvaccinated (6.1%)
patients required hospitalization for the progression of respiratory
insufficiency (p=0.23). A total of 4 vaccinated (4.9%)
and 8 unvaccinated (8.2%) patients were hospitalized for the
advancement of chronic diseases during SARS-CoV-2 infection
(p=0.07). The mean time to administration of symptoms were
6.01 ± 0.3 days in the group of vaccinated patients and 5.52
± 0.28 days in the group of non-vaccinated The mean time to
the resolution of symptoms were 4.37 ± 0.62 days in the group
of vaccinated patients and 3.83 ± 0.3 days in the group with
non-vaccinated patients (Table 2).
|
Vaccinated patients after MAB administration n=82 |
Non-vaccinated patients after MAB administration n=98 |
P values (without deviation) |
| Age (mean ± /- SEM) |
71.42 ± 1.33 |
70.23 ± 1.14 |
0.56 |
| Sex (males) |
31 (37.8%) |
34 (34.7%) |
0.25 |
| Day of the onset of the symptoms |
6.01 ± 0.3 |
5.52 ± 0.28 |
0.50 |
| Day of the positive antigen test |
4.63 ± 0.27 |
4.16 ± 0.21 |
0.61 |
| Day of the symptoms relieve after MAB |
4.38 ± 0.62 |
3.84 ± 0.3 |
0.33 |
| COVID-19 associated BRPN |
4 (4.9%) |
9 (9.2%) |
0.13 |
| Hospital admission due to BRPN |
3 (3.7%) |
6 (6.1%) |
0.23 |
| Hospital admission due to other causes |
4 (4.9%) |
8 (8.2%) |
0.07 |
| Long COVID |
12 (14.6%) |
29 (29.6%) |
0.02 |
Table 2: Vaccinated and unvaccinated patients after MAB administration.
Although there were numerically exciting findings in comparison
between groups of vaccinated and unvaccinated patients
in developing bronchopneumonia and need for admission to
hospital due to other causes such as destabilization of diabetes
mellitus or arterial hypertension, we proved the level of significance
in administering long-COVID syndrome (p=0.02). One
patient had a cytokine release syndrome-grade 3 requiring hospitalization.
We noted the presence of various comorbidities in
the patients; for example, the most common were cardiovascular
diseases such as a previous myocardial infarction or arterial
hypertension. Furthermore, there were type 1 or 2 diabetes
mellitus, oncological diseases, most often hemato-oncological
and uro-oncological diseases, chronic kidney disease in several
patients with the need, and conditions associated with immunodeficiency,
including induced immunodeficiency after solid
organ transplantation. In the group of vaccinated and unvaccinated
patients, the presence of obesity and cardiovascular diseases
(overcame myocardial infarction, arterial hypertension
with organ complications) showed the highest coincidence
with a longer recovery time, which can be considered as independent risk factors (Pearson coefficient 0.611) [7] (Figure 1).
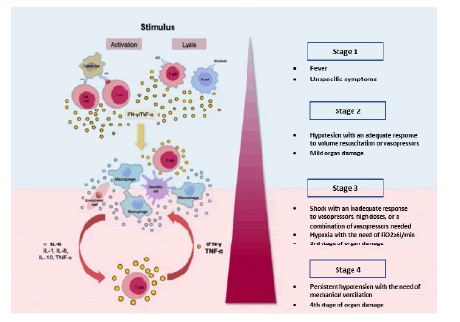
Figure 1: Activation and stages of the cytokine release syndrome. Reprinted from: Shimabukuro-Vornhagen et al. (2018). IL-6-Interleukin 6, IL-8-Interleukin 8, IL-10-Interleukin 10, TNFα-Tumor necrosis factor α, IFN-γ-Interferone γ.
Discussion
In this retrospective study, we found that compared to vaccinated
patients, a significant proportion of unvaccinated patients
developed long-COVID syndrome or persistent post-COVID syndrome
after receiving MAB (p=0.02). In the case of long-COVID,
these syndromes are manifested by the persistence of typical
symptomatology (dry cough, sub febrile, loss of smell or taste,
weakness, brain fog, and many others) from the acute illness
for four weeks and in the case of persistent post-COVID syndrome
for up to several months. Most often, we noticed the
loss of smell and taste or their change, as well as eating disorders,
persistent cough, and weakness or so-called brain fog,
which prevented the performance of everyday activities.
Several studies mention that post-COVID or long-COVID syndrome
symptoms seem pretty consistent, with a higher incidence
in women (14.9% compared to men 9.5%), increasing
with age (age over 70 years, p=0.0005). Other factors include
obesity, asthma, poor general health, poor pre-pandemic mental
health, and poor socio-demographic characteristics. The
impact of nationwide lockdowns, work-from-home, and restrictions
on physical activity on the rising proportion of the
already obese population with incorrect diet and physical activity
patterns is particularly noteworthy [8-15]. A prospective
study from Wuhan, including 1733 patients after overcoming
COVID-19 with a 6-month follow-up, showed up to 26% incidence
of sleep difficulties and 23% incidence of anxiety and depression
episodes. Even though the cause of long-COVID is still
not fully understood, it still affects infrastructure worldwide.
Furthermore, we found that patients with COVID-19 were
more often hospitalized for exacerbation of chronic diseases (4
vaccinated and 8 unvaccinated patients) than for progression
of COVID-19-associated BRPN (3 vaccinated and 6 unvaccinated
patients). The difference in the number of hospitalizations
in patients after and without vaccination could be caused by
a higher number of comorbidities and often a combination of
more comorbidities in the group of patients after vaccination than in non-vaccinated patients (p=0.07).
Cytokine release syndrome as an adverse effect of Casirivimab/
Imdevimab recombinant monoclonal antibodies is a product of
immune hyper activation and loss of regulation of the balance
between pro-inflammatory cytokines. A study by Leisman et al.
reported that elevation of inflammatory cytokines in patients
with severe to critical course of COVID-19 disease (including
height of IL-6) showed lower values than in patients with Acute
Respiratory Distress Syndrome (ARDS) in patients without
SARS-CoV-2 and in sepsis. In contrast, patients with COVID-19
have higher levels of D-dimers and C-reactive protein than other
critically ill patients. The authors conclude that the overall
critical course in patients with COVID-19 and elevated cytokine
levels have an unclear cause. Therefore, it is necessary to consider
alternative mechanisms of organ dysfunction [9].
The authors of the study Eskazan et al., present the results of
administering tocilizumab (a human chimeric monoclonal antibody
against IL-6) in patients with a severe course of COVID-19
accompanied by CRS, which is considered the leading cause
of mortality. The monitored patients observed an increase in
the levels of various indicators in a pro-inflammatory state (increase
in C-reactive protein, procalcitonin, D-dimer, and others).
Furthermore, early administration of tocilizumab, even
before placing the patient in an Intensive Care Unit, showed
success. Still, in patients with a severe course of COVID-19 and
with COVID-19-associated BRPN, the administration of tocilizumab
showed no benefit [10]. However, in the extensive randomized
controlled trial RECOVERY (Randomized evaluation
of COVID-19 therapy), tocilizumab demonstrated an improvement
in survival and clinical status in patients with a severe
course of COVID-19 with hypoxia and CRS, including a lower
rate of use of invasive mechanical ventilation (p=0.0001) [11].
Although very rarely, allergic reactions have been observed
with MAB Casirivimab/Imdevimab. These events occurred
within one hour after the end of the infusion. They resolved
after the administration of supportive treatment (e.g., volume therapy in combination with oxygen therapy and the use of antihistamines
without the need for subsequent hospitalization,
CRS 1-2). In addition, infusion-related reactions have occurred
with administering various infusion preparations containing
the combination of Casirivimab and Imdevimab. The manifestations
and symptoms of these reactions often included
tremors, dizziness/syncope, hot flashes, nausea, and urticaria.
In general, reactions occurred during or within 24 hours of infusion,
were mild to moderate in severity, and resolved with
over-the-counter medications (e.g., antihistamines, nonsteroidal
anti-inflammatory drugs) or without intervention [4].
Collective Ganesh et al. participated in a study with the administration
of MAB Casirivimab/Imdevimab in a total of 3596
patients. Patients had atleast one comorbidity that classified
them in the group with an increased risk of a severe course of
COVID-19. The collective identified groups of patients at higher
risk of needs to visit the emergency room or hospitalization
(chronic kidney disease, exacerbation of chronic lung disease)
or hospitalization in the intensive care unit (cardiovascular
complications). A lower risk of hospitalization has been reported
in patients with a higher body mass index (BMI) [12].
A study by Abani et al. reports the average onset time of symptoms
in patients with COVID-19 to be nine days on average. The
p-value for the difference in patient mortality between those
who received MAB and those who did not receive MAB was
p=0.14; in percentage terms, the mortality of patients after
MAB was 19%, and for patients without MAB, 21%. Despite the
administration of MAB, 31% of patients required intensive care
unit stay, and 12% of patients needed mechanical ventilation
support vs. 37% of patients without MAB in ICU and 14% with
mechanical ventilation support [13].
Conclusion
This retrospective study confirms a reduced incidence of bronchopneumonia
associated with SARS-CoV-2 in patients belonging
to risk groups of the population who were administered
MAB, especially in patients who previously received any of the
preparations of the vaccine regimens against COVID-19. We
also found a lower rate of hospitalization for bronchopneumonia
associated with COVID-9 in the vaccinated group. In addition,
we observed a statistically significant difference between
the groups of vaccinated and unvaccinated patients describing
the long-covid syndrome in favor of the group after vaccination
against COVID-19. Overall, casirivimab/imdevimab is a promising
treatment option for high-risk patients with mild to moderate
COVID-19, and its use should be encouraged in appropriate
patient populations.
Acknowledgement
None.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, et al. (2020) Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 382(24):2302-2315.
[Crossref] [Google Scholar]
- Lavezzo E, Franchin E, Ciavarella C, Cuomo-Dannenburg G, Barzon L, et al. (2020) Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 584(7821):425-429.
[Crossref] [Google Scholar]
- Lee S, Kim T, Lee E, Lee C, Kim H, et al. (2020) Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in Korea. JAMA Intern Med. 180(11):1447-1452.
[Crossref] [Google Scholar]
- UK Medicines & Healthcare Products Regulatory Agency. Casirivimab/imdevimab (Ronapreve): UK summary of product characteristics. 2021.
- Hansen J, Baum A, Pascal KE, Russo V, Giordano S, et al. (2020) Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 369(6506):1010-1014.
[Crossref] [Google Scholar]
- World Health Organization (WHO) Tracking SARS-CoV-2 variants.
- Timur, Luckhurst K (2022) Covariance calculator.
- Jeremy stangroom. Mann-whitney u test calculator. Social science statistics.
- Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, et al. (2020) Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 8(12):1233-1244.
[Crossref] [Google Scholar]
- Eşkazan AE, Balkan İİ, Demirbaş KC, Muhlis CA, Karaali R, et al. (2021) Tocilizumab in COVID-19: The Cerrahpaşa-PREDICT score. J Infect Chemother. 27(9):1329-1335.
[Crossref] [Google Scholar]
- RECOVERY Collaborative Group (2022) Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomized, controlled, open-label, platform trial. Lancet. 399(10325):665-676.
[Crossref] [Google Scholar]
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, et al. (2018) Cytokine release syndrome. J Immunother Cance. 6(1):56.
[Crossref] [Google Scholar]
- Ganesh R, Philpot LM, Bierle DM, Anderson RJ, Arndt LL, et al. (2021) Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis. 224(8):1278-1286.
[Crossref] [Google Scholar]
- Ministerstvo zdravotníctva Slovenskej republiky. Národné centrum zdravotníckych informácií Slovenskej republiky.
- Our World in Data. Coronavirus Pandemic (COVID-19).
Citation: Belian?inová M, Kleinová P, Gra?ák K, Vnu?ák M, Kolorz M, et al. (2023) Recombinant Human Monoclonal Antibodies
Casirivimab/Imdevimab use in Inhibition of the Sars-Cov-2 Virus Infection. J Healthc Commun. 8:80018.
Copyright: © 2023 Belian?inová M, et al. This is an open-access article distributed under the terms of the Creative Commons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author
and source are credited.