Keywords
Lead; Biosorbent; Kinetic parameters; Biosorption capacities; Delonix
regia pod
Introduction
Toxic Heavy metals can be released into water through metal
smelters, effluents from plastics, textiles, There is evidence that
present in the environment, even in low concentration of heavy metals cause dermal damage and cancer [1,2]. Lead is considered
as the most toxic metal exists in several industrial wastes, such
as chemicals, lead acid storage batteries. Lead poisoning in
human causes damaging to the kidneys, liver, and brain [2,3].
The removal of heavy metal from contaminated sites is very
important to restore ecosystem functions and stability [4]. The
search for low-cost techniques to remove heavy metals from
waste water using agricultural materials such as Maize leaves,
loquat leaves (Eriobotrya japonica), Psidium guajava leaves,
Scolymushis panicus, Azadirachta indica (Neem leaves), Ulmus
leaves, Oleaeuropaea (Olive leaves), and Prunusvium leaves [5],
rice straw, rice bran, rice husk, hyacinthroots, neem leaves [6]. Delonix regia is a species of flowering plant in the family Fabaceae,
subfamily Caesalpinioideae [7]. Delonix regia possesses several medicinal characters [8]. The effect Delonix regia biosorbent for
the removing of Methylene Blue dye [9], Hg (II) ion from water
[10] and Pb, Cu and Co ions [11]. Chemical treatment of biomass
with NaOH and citric acid increases its cation uptake ability as
the carboxyl groups of the biomass increases [12] Ion-exchange
has been suggested as one the mechanisms for heavy metal removal from aqueous solution [2,13]. This study can introduce
an economic value biosorbent for removing of toxic heavy metal
by using nanosized Delonix regia pod.
Experimental
From Sigma-Aldrich, Pb(NO3)2, HCl, citric acid and NaOH were
purchased .
Sample collection
The pods of Delonix regia were obtained from Shandawil Research
Station, Agriculture Research Center, Sohag, Egypt.
Instruments
Nano size of the investigated biosorbent was obtained by using
Retsch Muhle Brinkann Spectro Mill MS Micro-Grinding Mixing.
Biosorbent was characterized by X-ray powder diffraction using
a Philips X'Pert PRO MPD. (EDAX) unit was used to analyse the
chemical composition of the synthesized nanostructures. Fieldemission
scanning electron microscopy was used for studying
the morphology of sample. Functional groups on the biosorbent
surface were detected by using (FT-IR, 2000, PerkinElmer). mVISE-
pH-temperature bench Meters was used to adjust pH of
the solutions. Transmission electron microscopy images were
obtained with a 2000 EX (II0 microscope (J E O L-Japan). A
shaker bath (Heidolph M R-3001) was used for shaking. E-B-A, 20
zentrifugen D78532 tuttlingen was used to centrifuge the sample
after the adsorption process. The concentration of Pb2+ ions was
determined using (AAS) (model PerkinElmer-Analyst, 200).
Sample pretreatment
Delonix regia pods were cleaned with water, and then dried. Delonix regia pods were grinded to obtain a fine powder. The fine
powder was used as biosorbent in the experiments.
Treatment of Delonix regia (DR) by Citric Acid
Chemical modification of nano sized powder Delonix regia (DR) using NaOH followed by citric acid treatment. The synthesis of
CADR was carried out as followed, 200 grams of the powder was
placed in 4 L of 0.1 N NaOH,
then was stirred at 300 rpm for 1 h at 23oC to remove base. The
powder was rinsed with water and added to 4 L of distilled water.
This biomass was mixed with citric acid (CA) in a ratio of 1.0 g
powder to 7.0 mL of CA (0.6 M). The acid/powder Slurry was
dried over night at 50°C and then heated to 120oC for 1.5 h. Citric
acid (CA) treated DR powder (CADR) was filtered and washed in a
Buchner funnel under vacuum with 150–200 mL of distilled water
per gram of the product to remove excess CA. This volume of
water was sufficient to remove un reacted CA since no turbidity
from lead citrate was observed when the washed powder was
suspended in 10 mL of water to which 10 mL of 0.1 M lead nitrate
was added. The modified powder was dried at 50oC overnight
[2,14,15].
Preparation of Solution
Aqueous solution of Pb2+ ions was prepared by weighing out 1.60
g of Pb(NO3)2 and dissolved in a 1000 ml volumetric flask with de-ionized water to obtain a 1000 mg/L concentration. Different
initial concentrations of Pb2+ ions were prepared by Dilution.
Batch Biosorption Experiments
Effect of concentration of metal ion
A total of 50 ml of Pb2+ ions solution of different concentrations
was added to 0.3 g of the Adsorbent in a flat bottle and then the
mixture was stirred for 1 hr on a shaker at 300 rpm.
Effect of pH
Experiments were carried out at different pH (2:10) and pH was
adjusted by using 0.1 M (NaOH) or 0.1 M (HCl). A total of 50 ml of
Pb2+ ions solution of concentration (20 mg/L) was added to 0.3 g
of the Adsorbent in a flat bottle, then the mixture was stirred for
1 hr on a shaker at 300 rpm.
Effect of dosage
In each biosorption experiment, 50 ml of Pb2+ ions solution of
concentration (20 mg/L) was added to different dosage of the
adsorbent in bottle and then the mixture was stirred for 1 hr on
a shaker at 300 rpm.
Effect of contact time
In the biosorption kinetics experiment, 0.2 L of Pb2+ ions solution
of different concentrations was added to 1.2 g of the adsorbent in
flat bottle and then the mixture was stirred for 1 hr on a shaker at
300 rpm and a contact time (20: 120) minutes with time interval
20 minutes.
Effect of temperature and determination of thermodynamic parameters
A total of 50 ml of different concentrations of Pb2+ ions solution was
added to 0.3 g of the adsorbent in bottle at different temperature
and then the mixture was stirred for 1 hr on a shaker at 300
rpm. Then the mixture was centrifuged and the concentration of
Pb2+ions was determined. Were calculated using the relationships
(1) and (2) [2,16,17] can be used to calculate ΔH, ΔS, and ΔG( the
thermodynamic parameters for the adsorption process.
lnb = ΔS°/R - ΔH°/RT (1)
ΔG° = ΔH°-T ΔS°(2)
Calculation of metal uptake
The Pb2+ions uptake at equilibrium was calculated by:
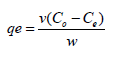 (3)
(3)
where qe is Pb2+ ions absorption capacity, v is the volume of the
Pb2+ ions solution and w is the amount of the adsorbent, Co and
Ce are initial Pb2+ ion concentrations and Ce are final (equilibrium)
Pb2+ ion concentrations. The efficiency of the Pb2+ ions removal
was also determined using;
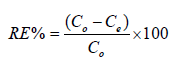 (4)
(4)
Where, RE% is the percentage of the removed Pb2+ions.
Kinetics study
The mechanism of the adsorption of Pb2+ions was studied using
pseudo first order kinetic models , the intraparticle diffusion and
pseudo second order kinetic models [2,18-20] and they are giving
in a linear form by Equations 5, 6 and 7, respectively
ln(qe-qt) = lnqe – k1t (5)
(t/qt)=1/ (k2q2e) + (t/qe) (6)
qt = kint t0.5 (7)
kinetic models are tested for suitability using correlation
coefficient (R2) [2,20,21].
Effect of chemical treatment
A total of 50 ml of Pb2+ ions solution of (20 mg/L) concentration
was added to 0.3 g of the chemically treatment adsorbent (CADR)
in bottle, then the mixture was stirred on a shaker for 1 hr at 300
rpm and the concentration of Pb2+ ions was determined .
Results and Discussion
Characteristics of the biosorbent
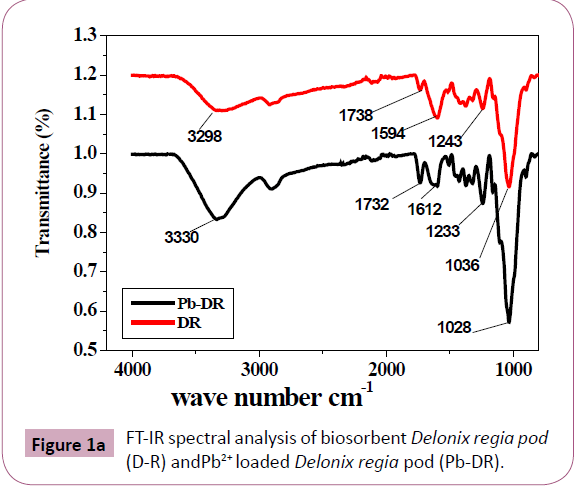
FTIR spectral analysis: FTIR spectral analysis of Delonix regia pod (DR) (Figure 1a) and Pb2+ ions loaded Delonix regia pod (Pb-
DR) (Figure 1a) were carried out. FTIR data of Delonix regia (DR)
indicates the functional groups. The main characteristic cellulose
peak appears in the region of 1000-1200 cm−1. [22]. The strong
and broad peak at 3298 cm−1, indicated the N-H bond of amino
groups and hydroxyl group. The shift in the peak to 3330 cm−1 in
the spectra of the metal loaded Delonix regia pod powder shows
the binding of Lead ions with hydroxyl and amino groups [23-25]
peak at 2916 cm−1 in the spectra of the Delonix regia pod powder
indicated CH3 and CH2
groups. The peak at 1594 cm−1 indicates
CO, OH and C-O groups, the Shift to 1612 cm−1 indicated the metal
binding. Band at 1036 cm−1 indicated the C-O of alcohols, the
shift to 1028 cm−1 indicated binding of Pb2+ ions with C-O group
[2,24-26]. Peak at 1738 cm−1, which is indicative of carbonyl
group, shifted to wave number of 1732 cm−1 after Pb2+ adsorption [2,27,28]. Band at 1243 cm−1 indicates carboxylic acids which
shifted to 1233 cm−1 after adsorption of Pb2+[29]. The shifts in
the absorption peaks indicate the binding of metal ions on the
surface of the powder.
Figure 1a:FT-IR spectral analysis of biosorbent Delonix regia pod (D-R) and Pb2+ loaded Delonix regia pod (Pb-DR).
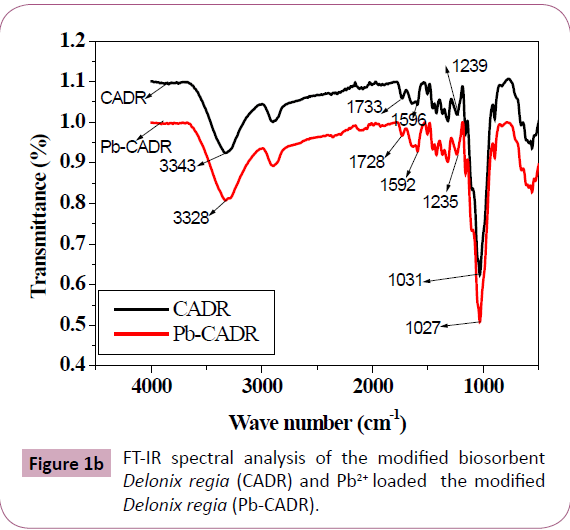
Figure 1b: FT-IR spectral analysis of the modified biosorbent
Delonix regia (CADR) and Pb2+ loaded the modified
Delonix regia (Pb-CADR).
Also FT-IR for detection the groups on the modified biosorbents
[Citric Acid (CA) treated DR powder (CADR) before and after the
biosorption of Pb2+ ions (Pb-CADR) was shown in Figure 1b.
Comparison of the IR spectra of samples of DR and CA modified
DR (CADR) revealed that a characteristic stretching vibration
absorption band of carboxyl group at 1733 cm−1 is present in the
IR spectrum of CADR samples. This indicates the esterification
between alcohol groups of cellulose in DR and citric acid
The broad absorptions around 2500-3500 cm−1 centered at
3343 confirm the existence of carboxylic OH groups and free
COOH groups after CA modification. It appears from Figure 1b that the different functional groups on CADR are responsible for
biosorption of Pb2+ A change in peaks position at 3328 cm−1 in
the spectrum of Pb2+ loaded CADR indicates the binding with
hydroxyl groups. The peak at 1733 cm−1 shifted to 1728 cm−1 in the
spectrum of Pb2+ loaded CAMO indicating the binding of metal ions to carboxylic groups also [2,30,31].
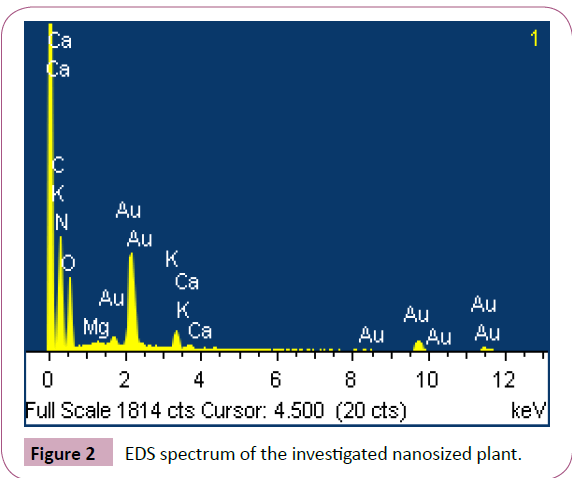
Elemental analysis: To determine the chemical composition
of the biosorbent. Elemental analysis of Delonix regia (pod) is
shown in Figure 2.
Figure 2: EDS spectrum of the investigated nanosized plant.
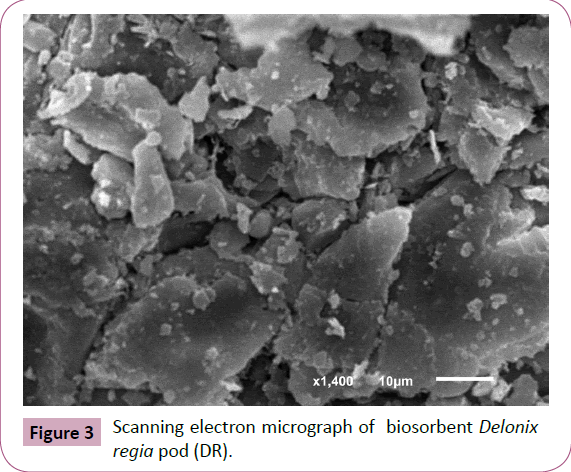
Scanning electron micrograph (SEM): SEM of biosorbent Delonix
regia pod(D-R) (Figure 3) are used to show the morphology
of Delonix regia pod, which exhibits the structure porosity of
biomass. The surface morphology of Delonix regia pod powder
showed that the powder was a fine particle. The particles have a
large number of steps and edges.
Figure 3: Scanning electron micrograph of biosorbent Delonix
regia pod (DR).
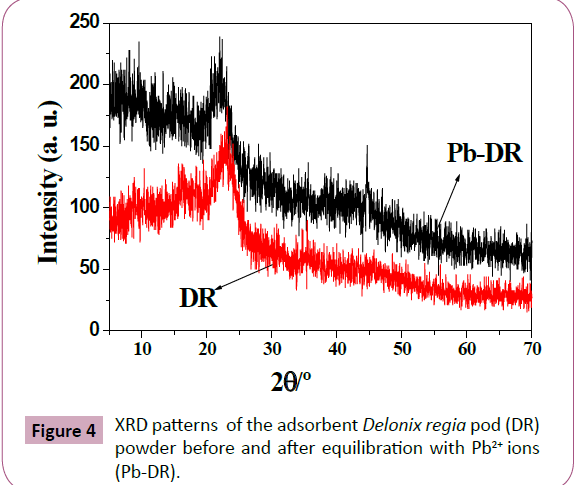
XRD analysis: XRD of the Delonix regia pod powder is shown
in Figure 4 indicates the amount of amorphous material in the
sample. XRD of the adsorbent Delonix regia indicate that the
structure of Delonix regia pod powder has a small different change due to the appearance of amorphous peak at 2θ=44.7
after adsorption process confirming adsorption of Pb2+ ions.
Figure 4: XRD patterns of the adsorbent Delonix regia pod (DR)
powder before and after equilibration with Pb2+ ions
(Pb-DR).
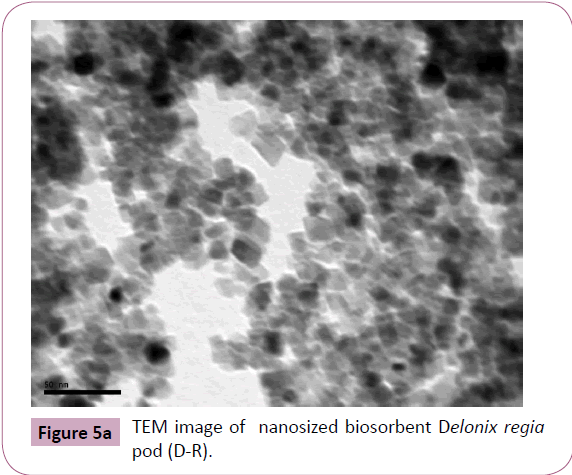
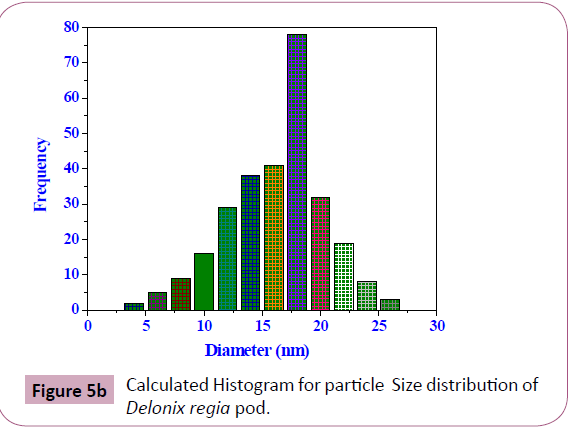
Transmission electron microscopy (TEM): The sample was
subjected to TEM analysis (Figure 5a) to indicate the particle
size and the major size of the particles was found to be 18 nm
(Figure 5b).
Figure 5a: TEM image of nanosized biosorbent Delonix regia
pod (D-R).
Figure 5b: Calculated Histogram for particle Size distribution of Delonix regia pod.
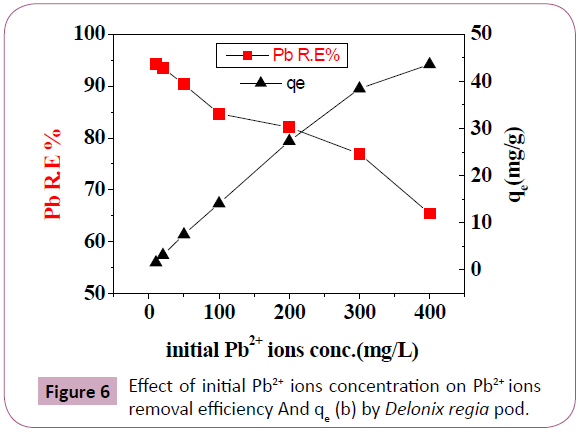
Effect of initial concentration: Figure 6 and Table 1 illustrated
the effect of metal ions concentration on Pb2+ ions biosorption
is in (qe) increases as the concentration rises, as Pb2+ ions are
more available for interaction with the biosorbent. The Pb2+ ions R. E for initial concentration 10 and 20 mg/L are 94.3%
and 93.5%, respectively and decreases as the concentration
increases. A greater chance was available for metal removal at
low concentrations, biosorption sites took up the available Pb2+ ions when increasing concentrations. So, initial concentration of
Pb2+ ions solutions increases the biosorption [2,32-34].
| Co (mg/L) |
Ce (mg/L) ± Sd |
Pb2+ ions R.E.% ± Sd |
qe(mg/g) ± Sd |
| 10 |
0.57 ±0.03 |
94.30 ±0.06 |
1.57 ±0.02 |
| 20 |
1.30 ±0.10 |
93.50 ±0.09 |
3.16 ±0.04 |
| 50 |
4.84 ±0.12 |
90.33 ±0.30 |
7.53 ±0.10 |
| 100 |
15.30 ± 0.13 |
84.70 ±0.40 |
14.12 ±0.13 |
| 200 |
35.88 ± 0.23 |
82.06 ±0.06 |
27.35 ±0.11 |
| 300 |
69.08 ± 0.20 |
76.97 ±0.08 |
38.49 ±0.07 |
| 400 |
138.28 ±0.34 |
65.43 ±0.23 |
43.62 ±0.21 |
Table 1: Pb2+ions Removal Efficiency and qe at different initial concentrations
Figure 6: Effect of initial Pb2+ ions concentration on Pb2+ ions
removal efficiency And qe (b) by Delonix regia pod.
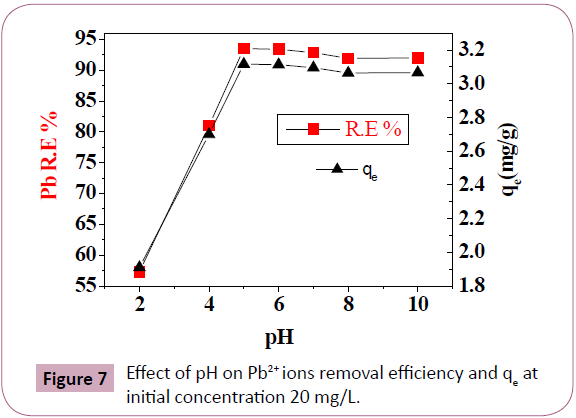
Effect of pH: Figure 7 and Table 2 illustrated the effect of pH of
a solution in the adsorption process. R.E. and qe increase as the
pH increase. The amount of Pb2+ ions removed by the Delonix
regia at low pH 2 was low (1.91 mg/g) and R.E. 57.3% compared
to the amounts removed at pH 4 to 10 were ranged from (2.7
mg/g and R.E. 81% at pH 4) to 3.12 mg/g and R.E. 93.5% at pH
5. Because at low pH the concentration of H+ is high [19], as H+ ions were being removed by the biosorbent, instead of the Pb2+ ions, [21,35] at higher concentration of H+ ions, the biosorbent
becomes more positive charge on the surface and the attraction
between biosorbent and Pb2+ ions is reduced [36]. At higher
pH the capacity of the adsorbent reduced, the reduction in
adsorption may be due to the increasing of OH- ions, or Pb2+ ions
were precipitated as lead hydroxide [2,37].
| pH |
Ce (mg/L) ± Sd |
Pb2+ ions R.E.%± Sd |
qe (mg/g) ± Sd |
| 2 |
8.54 ± 0.09 |
57.30 ± 0.12 |
1.91 ± 0.04 |
| 4 |
3.80 ± 0.05 |
81.00 ± 0.08 |
2.70 ± 0.07 |
| 5 |
1.30 ± 0.02 |
93.50 ± 0.32 |
3.12 ± 0.03 |
| 6 |
1.32 ± 0.04 |
93.40 ± 0.07 |
3.11 ± 0.06 |
| 7 |
1.430.02 |
92.85 ± 0.61 |
3.10 ± 0.01 |
| 8 |
1.62 ± 0.013 |
91.92 ± 0.18 |
3.06 ± 0.02 |
| 10 |
1.60 ± 0.01 |
92.00 ± 0.43 |
3.07 ± 0.03 |
Table 2: Pb2+ ions removal efficiency qe at initial concentration of 20 mg/L at different pH values
Figure 7: Effect of pH on Pb2+ ions removal efficiency and qe at
initial concentration 20 mg/L.
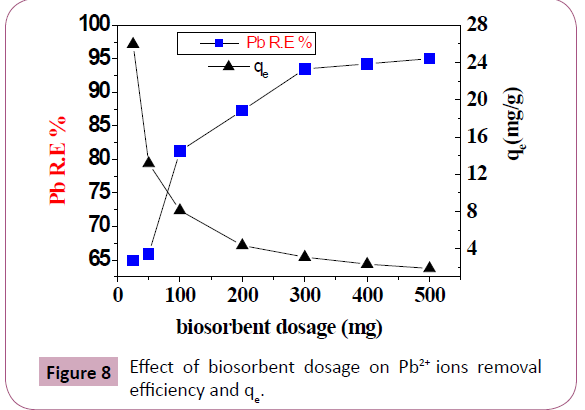
Effect of biosorbent dosage: It is an effective factor to study the
capacity of a biosorbent. R.E. increases with least value of 64.65%
obtained with 25 mg and highest value of 95.04% with 500 mg of
the biosorbent, this because at high dosage, there is an increase in
surface area and availability of biosorption sites, but qe decreases
as a decrease in the amount of Pb2+ ions adsorbed per unit weight
of biosorbent [2,38-40]. These results are illustrated in Figure 8 and Table 3.
| qe(mg/g) |
Pb2+ ions R.E.% ± Sd |
Ce(mg/L) ± Sd |
Biosorbent |
| ± Sd |
Dosage(mg) |
| 25.98 ± 0.20 |
64.95 ± 0.33 |
7.01 ± 0.09 |
25 |
| 13.18 ± 0.13 |
65.90 ± 0.41 |
6.82 ± 0.05 |
50 |
| 8.12 ± 0.09 |
81.24 ± 0.09 |
3.75 ± 0.02 |
100 |
| 4.37 ± 0.07 |
87.30 ± 0.012 |
2.54 ± 0.04 |
200 |
| 3.12 ± 0.04 |
93.50 ± 0.07 |
1.30 ± 00.01 |
300 |
| 2.36 ± 0.02 |
94.25 ± 0.16 |
1.15 ± 0.02 |
400 |
| 1.91 ± 0.01 |
95.04 ± 0.07 |
0.99 ± 0.01 |
500 |
Table 3: Pb2+ ions removal efficiency and qe at different biosorbent dosage
Figure 8: Effect of biosorbent dosage on Pb2+ ions removal
efficiency and qe.
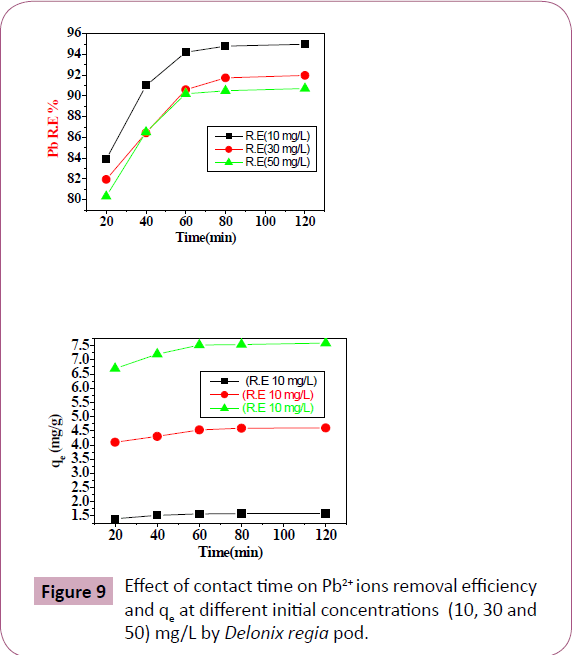
Effect of contact time: Table 4 and Figure 9 illustrated the effect
of contact time for the adsorption of Pb2+ ions by Delonix regia.
The amount of Pb2+ ions absorbed increased with an increase
in the contact time and reach equilibrium in 60 minutes. This
because long contact time and availability of active sites, it was
followed by a reduction in the metal uptake. There was a slightly
increasing or remain constant in the Pb2+ ions removal, as the
sites are less available [2,41,42].
| Time (min) |
Pb2+ R.E.% at Co(10) |
Pb2+ R.E.% at Co(30) |
Pb2+ R.E.% at Co(50 ) |
qt at |
qt at |
qt at |
Ct at |
Ct at |
Ct at |
| Co(10) |
Co(30) |
Co(50) |
Co(10) |
Co(30) |
Co(50) |
| 20 |
83.9 |
81.95 |
80.32 |
1.4 |
4.1 |
6.69 |
1.61 |
5.41 |
9.84 |
| 40 |
91 |
86.43 |
86.51 |
1.52 |
4.3 |
7.2 |
0.9 |
4.1 |
6.74 |
| 60 |
94.2 |
90.6 |
900 |
1.57 |
4.53 |
7.52 |
0.57 |
2.81 |
4.9 |
| 80 |
94.8 |
91.73 |
90.49 |
1.58 |
4.59 |
7.54 |
0.52 |
2.5 |
4.75 |
| 120 |
94.97 |
91.97 |
90.71 |
1.58 |
4.6 |
7.59 |
0.5 |
2.4 |
4.65 |
Table 4: Effect of contact time on Pb2+ ions removal efficiency and qe at different initial concentrations (10, 30 and 50) mg/L by Delonix regia pod
Figure 9: Effect of contact time on Pb2+ ions removal efficiency
and qe at different initial concentrations (10, 30 and
50) mg/L by Delonix regia pod.
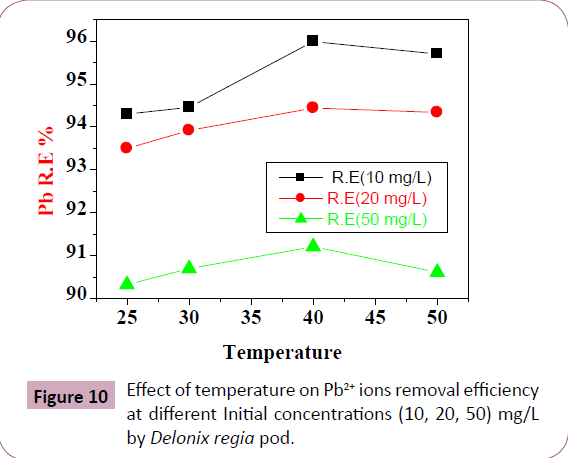
Effect of temperature: Table 5 and Figure 10 illustrated the effect
of the temperature on adsorption, the Pb2+ R.E. and qe by Delonix
regia increases while the temperature is increasing, as the active
sites have increased and encourages the process of biosorption,
due to increase in the movement of the Pb2+ ions and pore size
indicating an endothermic process [2,43-45].
Temp
( oC) |
Pb2+ R.E.%
at Co(10 ) |
Pb2+ R.E.%
at Co
(20) |
Pb2+ R.E.%
atCo
(50 ) |
qe at Co(10) |
qe at
Co(20) |
qe at
Co(50) |
Ce at
Co(10) |
Ce at Co(20) |
Ceat
Co( 50) |
| 25 |
94.30 |
93.50 |
90.33 |
1.57 |
3.17 |
7.53 |
0.57 |
1. 30 |
4.84 |
| 30 |
94.46 |
93.92 |
90.70 |
1.57 |
3.13 |
7.56 |
0.55 |
1.22 |
4.65 |
| 40 |
95.99 |
94.44 |
91.21 |
1.60 |
3.15 |
7.60 |
0.41 |
1.11 |
4.40 |
| 50 |
95.70 |
94.34 |
90.62 |
1.59 |
3.14 |
7.55 |
0.43 |
1.13 |
4.69 |
Table 5: Effect of temperature on Pb2+ ions removal efficiency and qe at differentinitial Concentrations (10, 20, 50) mg/L by Delonix regia pod
Figure 10: Effect of temperature on Pb2+ ions removal efficiency
at different Initial concentrations (10, 20, 50) mg/L
by Delonix regia pod.
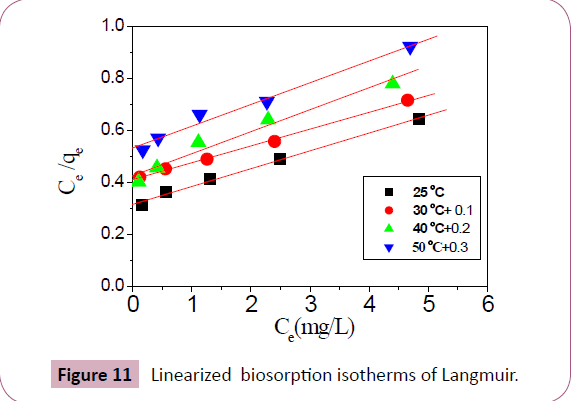
Adsorption isotherm: Pb2+ ions distribution between the solid and
liquid phases can be described by the Freundlich and Langmuir isotherms [46] qe increased with the initial concentration of Pb2+ as expected [47,48]. qm is 15.26 mg/g of Delonix regia. Langmuir
model suggests that the adsorption take places on homogeneous
sites. Langmuir isotherm equation is represented by equation 8
in a linear form [2,49].
 (8)
(8)
Plot of Ce /qe against Ce give a line with intercept 1/qm b and
slope 1/qm is obtained (Figure 11), which shows Lead biosorption
isotherms of Langmuir. From the intercept and slope the Langmuir
parameters (b and qm) are calculated. These values may be used
for compared and correlate the biosorptive properties of Delonix
regia of the Freundlich has the linear form [2,50].
Figure 11: Linearized biosorption isotherms of Langmuir.
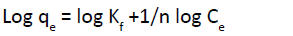 (9)
(9)
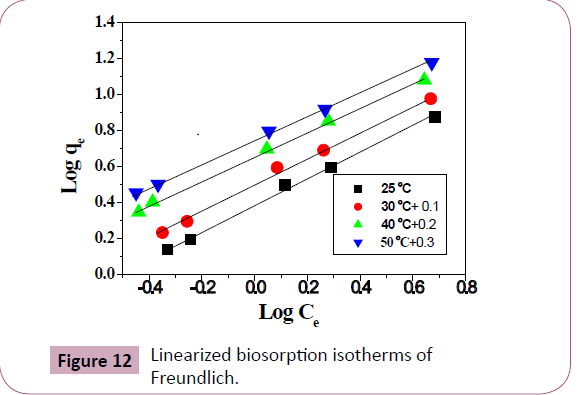
From a plot, a line with slope and intercept 1/n and log Kf respectively is obtained (Figure 12). The slope, 1/n , indicate the
intensity of adsorption and log Kf indicate the adsorption capacity [51] parameters of Pb2+ ions adsorption was given in Table 6a dimensionless constant separator factor (RL) can classify the
Isotherms [52] stated as:
| T (K) |
Langmuir |
Friendlich |
qm (mg/g)
± Sd |
b(L/mg)± Sd |
R2 |
n± Sd |
1/n |
Kf(mg/g)
± Sd |
R2 |
| 298 |
15.26 ± 0.18 |
0.200 ± 0.01 |
0.995 |
1.377 ± 0.09 |
0.726 |
2.46±0.01 |
1 |
| 303 |
15.41 ± 0.09 |
0.207 ± 0.02 |
0.994 |
1.372 ± 0.01 |
0.73 |
2.53±0.09 |
0.999 |
| 313 |
12.99 ± 0.23 |
0.315 ± 0.06 |
0.983 |
1.520 ± 0.06 |
0.657 |
2.91±0.03 |
0.999 |
| 323 |
12.56 ± 0.35 |
0.317 ± 0.05 |
0.991 |
1.540 ± 0.07 |
0.65 |
2.81±0.05 |
0.998 |
Table 6: Isotherm constants of Pb2+ ions biosorption on Delonix regia pod at various temperatures.
Figure 12: Linearized biosorption isotherms of
Freundlich.
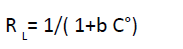 (10)
(10)
RL Mathematical calculation indicates the shape of isotherm
, irreversible if (RL=0), linear if (RL=1), unfavorable if (RL>1)
favorable if (0<RL<1). RL values have arrange from 0.059 to
0.333 (Table 7) n values were greater than 1 [53], these values
indicating a formation of a bond between Pb2+ ions and adsorbent
and indicating favorable biosorption. This indicate that Pb(II) ions
adsorption on Delonix regia is favorable. Linearity coefficient
(R2) can be used to examine the fitting of the models. According
to linearity coefficients (R2=1) Freundlich models has a good
fit models and adsorption of Lead ion on Delonix regia follow
Freundlich isotherm models.
| Co(mg/L) |
RL at
25o |
RL at
30o |
RLat
40oC |
RL at
50oC |
| 10 |
0.333 |
0.326 |
0.241 |
0.239 |
| 20 |
0.200 |
0.195 |
0.137 |
0.136 |
| 50 |
0.091 |
0.088 |
0.060 |
0.059 |
Table 7: A dimensionless constant separator factor (RL) for Langmuir type
biosorption process.
Thermodynamic studies
From a plot lnb against 1/T, thermodynamics equilibrium constant
was used to obtain the other thermodynamic parameters. The
biosorption capacity of the Delonix regia for Lead increased as
temperature increased, indicating the adsorption process was endothermic. Thermodynamic parameters (ΔG,° ΔS° and ΔH°) were
determined using the equations (1), (2) [54], the slope ΔH°/R, and
intercept ΔS°/R are obtained and the values of ΔH° and ΔS° were
calculated (Table 8). The adsorption process of Lead ions on the
Delonix regia was endothermic as ΔH° values were Positive. a
positive ΔG° value suggested an ion-exchange mechanism occur
in the biosorption of Pb2+ and a complex formed by Pb2+ with
the Delonix regia [2,55] An increase in randomness during the
biosorption as the Positive ΔS◦ value [2,56-58].
|
|
|
|
| Temperature (K) |
ΔG° (KJ/mol) |
ΔH°(KJ/mol) |
ΔS° (J/mol. K) |
| 298 |
4.73 |
17.69 |
43.84 |
| 303 |
4.51 |
| 313 |
4.08 |
| 323 |
3.64 |
Table 8: Thermodynamic parameters for the biosorption process
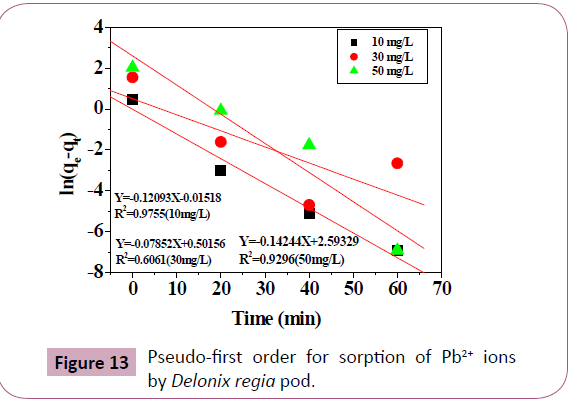
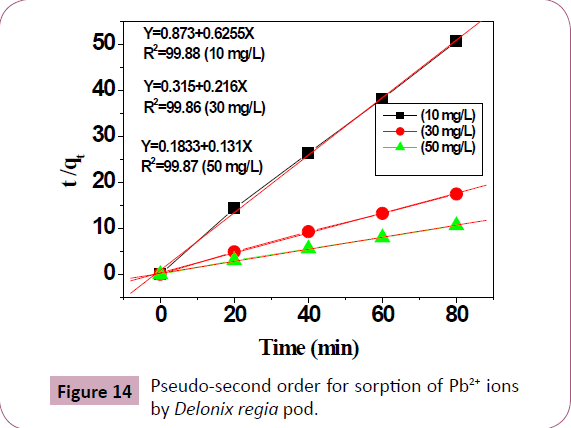
Kinetic studies on the biosorption of Pb2+ ions
Pseudo first order, pseudo second order kinetic and the intra
particle diffusion models can be used to test the mechanism of the
adsorption of metal ions [2,19-21]. The adsorption kinetic of the
adsorbed Pb2+ ions was studied (Figures 13 and 14). Correlation
coefficient, R2 can be used to test the suitability of these models
[2,21] The variable and constant of each kinetic model were
calculated and were presented in Table 9, the calculated qe determined from the plot of the pseudo first order model differs
from the experimental qe. This indicates that pseudo first order
model is not good in studying the kinetics of the biosorption of
Pb2+ ions compared to pseudo second order models (R2=0.999) for
Pb2+ ions, Table 9. So the second order kinetics is good in studying the kinetics of the biosorption of Pb2+ ions, as the calculated qe
(7.54 mg/g are very close to the experimental qe (7.6 mg/g),
suggesting that the biosorption of the Pb2+ ions solutions involves
the Pb2+ ion and the Delonix regia biosorbent particles [2,58,59].
| Co (mg/L) |
Pseudo-first order |
Pseudo-second order |
|
|
| Intraparticle diffusion |
Observed qe |
| |
(mg/g) |
| k1 |
qe |
R2 |
k2 |
qe(mg/g)± Sd |
R2 |
Kint |
C |
R2 |
± Sd |
| (1/min) |
(mg/g) |
(g/mg.min)± Sd |
|
| ± Sd |
± Sd |
|
|
| 10 |
0.083 ± 0.005 |
1.36 ± 0.06 |
0.985 |
0.448±0.01 |
1.6±0.03 |
0.9988 |
0.027 |
0.57 |
0.755 |
1.58±0.02 |
| 30 |
0.069 ± 0.003 |
3.41 ± 0.07 |
0.956 |
0.148±0.03 |
4.6±0.05 |
0.9986 |
0.818 |
2.81 |
0.861 |
4.59±0.06 |
| 50 |
0.091 ± 0.009 |
7.23 ± 0.12 |
0.972 |
0.094±0.01 |
7.6±0.09 |
0.9987 |
0.137 |
4.9 |
0.807 |
7.54±0.10 |
Table 9: Kinetic parameters of Pb2+ ions biosorption at different initial concentration.
Figure 13: Pseudo-first order for sorption of Pb2+ ions
by Delonix regia pod.
Figure 14: Pseudo-second order for sorption of Pb2+ ions
by Delonix regia pod.
Effect of chemical treatment of the biosorbents on biosorption efficiency
The effect of chemical treatment of the biosorbents by esterifying
with NaOH followed by citric acid treatment (CADR) on the R. E
compared with (DR) was studied and shown in Table 10. It was
observed that the R.E.% of metal ions by (CADR) was higher than
the R.E.% of metal ions by (DR) and this was due to the Chemical
treatment of biomass with NaOH and citric acid increases its
cation uptake ability as the carboxyl groups of the biomass
increases [2,31,32].
| Biosorbent |
Delonix regia |
Co (20 mg/L)
of metal ions |
DR |
CADR |
| Ce (mg/L) ± Sd |
R.E.% ± Sd |
Ce (mg/L) ± Sd |
R.E.% ± Sd |
| Cd2+ |
1.52± 0.02 |
92.42± 0.19 |
0.259± 0.03 |
98.71± 0.15 |
| Pb2+ |
1.30± 0.06 |
93.50± 0.11 |
0.390± 0.01 |
98.05± 0.17 |
Table 10: Effect of chemical treatment of Biosorbent on biosorption
efficiency.
Conclusion
1. Nano size of Flamboyant Pod (Delonix regia) was used for biosorption
of toxic Pb2+ ions from solution and is consider a very effective biosorbent
in the removal of heavy metals. This study indicated that: The Adsorption
process depends on temperature, pH, Contact time, dosage and metal
ion concentration.
2. Adsorption of Pb2+ ions from solutions obeyed Freundlich isotherm
models. qm of Pb2+ ions on Delonix regia is 43.62 mg g−1.
3. The biosorption process was endothermic an ion-exchange
mechanism applies in the biosorption of (Pb2+ ions). This confirmed by
thermodynamic studies.
4. Second order kinetics models is a better than the pseudo first order in
studying the kinetics of the biosorption of Pb2+ ions.
References
- Abdel-RahmanLH, Abu-Dief AM, Abd- El Sayed MA, Zikry MM (2016) Chemistry and Materials Research 8: 8-22.
- Agarwal S, Tyagi I, Gupta VK, Dehghani MH, Jaafari J,et al. (2016) Rapid removal of noxious nickel (II) using novel γ-alumina nanoparticles and multiwalled carbon nanotubes: Kinetic and isotherm studies. J Mol Liquids 224: 618-623.
- Aharoni A, Ungarish M (1977) Kinetics of activated chemisorption part 2. Theoretical models. J Chem Soc Faraday Trans73:456-464.
- Aksu Z (2001)Biosorption of Reactive Dyes by Dried Activated Sludge: Equilibrium and Kinetic Modeling. Biochem Eng J 7:79-84.
- Ali EN, Alfarra SR, Yusoff MM, Rahman MLR (2015) Environmentally Friendly Biosorbent from Moringa oleifera Leaves for Water Treatment. IJESD 6: 165-169.
- Azarpira H, Mahdavi Y, Balarak D (2016) Removal of Cd(II) by adsorption on agricultural waste biomass. Der Pharma Chemica 8: 61-67.
- Balarak D, Azarpira, H, Mostafapour FK (2016) Thermodynamics of removal of cadmium by adsorption on Barley husk biomass Der Pharma Chemica 8: 243-247.
- Balarak D, Joghataei A, Azarpira H, Mostafapour FK (2016)Isotherms and thermodynamics of Cd (II) Ion Removal by adsorption onto Azolla Filiculoides. IJPT15780-15788.
- Balarak D, Yari AR, Mostafapour FK, Mahdavi Y, Joghataei A (2016) Agricultural Waste as Adsorbent for Removal of Chromium (VI) from Aqueous Solution. Arch Hyg Sci 5: 310-318.
- Bhatti HN, Khalid R, Hanif MA (2009) Dynamic biosorption of Zn (II) and Cu (II) using pretreated Rosa gruss an teplitz (red rose) distillation sludge. Chem Eng J 148: 434-443.
- Bhatti HN, Nasir AW, Hanif MA (2010) Efficacy of Daucuscarota L waste biomass for the removal of chromium from aqueous solutions.Desalination 253:78-87.
- Boparai HK, Joseph M, O'Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nanozerovalent iron particles. J Hazard Mater 186: 458-465.
- Burke D(2005)The complete Burke's backyard: the ultimate book of fact sheets. Murdoch Books p: 269.
- Corupcioglu MO, Huang CP (1987)The Adsorption of Heavy Metals onto Hydrous Activated Carbon. Water Research 21:1031-1044.
- Donmez GC Aksu Z, Ozturk A, Kutsal TA (1999)Comparative Study on Heavy Metals Biosorption of Some Algae. Process Biochem34: 885-892.
- Dursun AY (2006) A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper and lead ions ontopretreated Aspergillusniger. Biochem Eng J 28: 187-195.
- Egila JN, Dauda BEN, Jimoh T (2010) Biosorptive removal of cobalt (II) ions from aqueous solution by Amaranthushydridus L stalk wastes. African J Biotechnol 9: 8192-8198.
- Farhan AM, Al-Dujaili AH, Awwad AM (2013) Equilibrium and kinetic studies ofcadmium(II) and lead(II) ions biosorption onto Ficus carcia leaves. Inter J Indust Chem 24.
- Farrokhzadeh H, Taheri E, Ebrahimi A, Fatehizadeh A, Dastjerdi MV, et al. (2013) Effectiveness of Moringa oleifera powder in removal of heavy metal from aqueous solution. Fresenius Environmental Bulletin J 22:1516-1523.
- Halpern SD, Ubel PA, Caplan AL (2002) Solid-organ transplantation in HIV-infected patients. N Engl J Med 347:284-287.
- Hasar H (2003) Adsorption of nickel (II) from aqueous solution onto activated carbon prepared from almond husk. J Hazard Mater B97: 49-57.
- Hashem MM, Elhmmali A, Ghith EE, Saad MM, Khouda (2007) Utilization of chemically modified Alhagiresidues for the removal of Pb (II) from aqueous solution. Energy Edu Sci Technol 20: 1-19.
- Ho YS, McKay G (1898) The Kinetics of Sorption of Divalent Metal Ions onto Spagnum Moss Peat. Water Res 34: 735.
- Ho YS (2006) Review of second order models for adsorption systems. JHazard Mater 136: 681-689.
- Hossain A, Bhattacharyya SR, Aditya G (2015) Biosorption of cadmium from aqueous solution by shell dust of the Freshwater snail Lymnaealuteola. Environ Technol Innovation 4: 82-91.
- Jain CK, Singhal DC, Sharma MK (2004) Adsorption of zink on bed sediment of River London: adsorption models and kinetics. J Hazard Mater B 114: 231-239.
- Jimoh TO, Iyaka YA, Nubaye MM (2012) Sorption Study of Co (II), Cu(II) and Pb(II) ions Removal from Aqueous Solution by Adsorption on Flamboyant Flower (Delonix regia). American J Chem 2: 165-170.
- Jimoh T, Egila JN, Dauda BEN, Iyaka YA (2011) Preconcentration and removal of heavy metal ions from aqueous solution using modified charcoal. J Environ Chem Ecotoxicol 3: 238-243.
- Juang LC, Wang CC, Lee CK (2006) Biosorption of Basic Dye onto MCM-41. Chemosphere 64: 1920-1928.
- Kumar KV, Ramamurthi V, Sivanesan S (2006) Biosorption of Malachite Green, a Cationic onto Pithophorasp, a Fresh Water Algae. Dye pigments 69: 102-107.
- Langergren S(1898) About the Theory of So-Called Biosorption of Soluble Substances. K Sven Vetenskapsakad handlingnaar Band 24: 1-39.
- Langmuir I (1918)The Adsorption of Gases on Plane Surfaces of Glass, Micaand Platinum. J Am Chem Soc 40: 1361-1403.
- Larous S, Meniai AH, Lehocine MB (2005)Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 185: 483-490.
- Levine NI (1998) Physical Chemistry, (4th edn), New York.
- Malakootian M, Fatehizadeh A, Yousefi N, Ahmadian M, Moosazadeh M (2011)Fluoride removal using Regenerated Spent Bleaching Earth (RSBE) from groundwater: Case study on Kuhbonan water. Desalination277: 244-249.
- Mariajancyrani J, Chandramohan G, Ravkumar R (2013) Iosolation and identification of phytoconstituents from Delonix regia leaves. Int J Pharm Sci 5:671-674.
- Marshall WE, Wartelle LH, Boler DE, Johns MM, Toles CA (1999) Bioresource Technol 69: 263-268.
- Mathew SB, Pillai AK, Gupta VK (2007) A Rapid Spectrophotometric Assay of Some Organo Phosphorus Pesticide Residues in Vegetable Samples. Spectrochimica Acta Part A 67: 1430-1432.
- Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005)Removal of heavy meal ions from aqueous solutions using carbon aeogel as an adsorbent. J Hazard Mater 122: 161-170.
- Minamisawa M, Minamisawa H, Yoshida S, Takai N (2004) Adsorption behavior of heavy metals on biomaterials. J Agric Food Chem 52: 5606.
- Namasivayam C, Yamuna RT (1995) Adsorption of chromium in tanned leather gloves and relapse of chromium allergy from tanned leather samples. Analyst 123: 935-937.
- Neto CP, Rocha J, Gil A, Cordeiro N, Esculcas AP (1995) 13C solid-state nuclear magnetic resonance and Fourier transform infrared studies of the thermal decomposition of cork. Solid State Nucl Mag 4: 143-151.
- Owoyokun TO (2009) Biosorption of Methylene Blue Dye Aqueous Solutions on Delonix regia (Flamboyant Tree) Pod Biosorbent. Pacific J Sci Technol 10: 872-883.
- Oyebamiji J, Babarinde NAA, Popoola AO, Oninla VO (2009)Kinetic, Equilibrium, and Thermodynamic Studies of the Biosorption of Pb(II) and Pb(II) from Aqueous Solutions by Talinumtriangulare (water leaf). Pacific J Sci Technol10: 428-436.
- Ozcan AS,Ozcan A (2004) Adsorption of acid dyes from aqueous solutions onto acid-activated bentonite.J Colloid Interface Sci 276: 39-46.
- Pons MN, Bonte SL, Potier O (2007) Spectral analysis and fingerprinting for biomediacharacterisation. J Biotechnol 113: 211.
- Ramakrishnan M, Sulochana N (2009) Utilisation of Flame Tree Waste Biomass for the Removal of Hg(II) from Water. Acta Chim Slov 56:282-287.
- Reddy DHK, Seshaiah K, Reddy AVR, Rao MM, Wang MC (2010)Biosorption of Pb2+ from aqueous solutions by Moringaoleifera bark: Equilibrium and kinetic studies. J Hazard Mater174: 831-838.
- Reddy DHK, Seshaiah K, Reddy AVR, Lee SM (2012) Carbohydrate Polymers 88: 1077-1086.
- Reddy DHK, HarinathY, Seshaiah K, Reddy AVR (2010) Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162: 626-634.
- Riaza M, Nadeema R, Hanifa MA, Ansaric TM,Rehmana KJ (2009) Pb(II) biosorption from hazardous aqueous streams using Gossypiumhirsutum (cotton) waste biomass. J Hazard Mater 161: 88.
- Singha S, Das SK (2012) Removal of Pb(II) ions from aqueous solution and industrial effluent using natural biosorbents. Environ Sci Pollut Res Int 19: 2212-2226.
- Srivastava SK, Singh AK, Sharma A (1994) Studies on the uptake of lead and zinc by lignin obtained from black liquoar: a paper industry waste material. Environ Technol 15: 353-361.
- Stephen IB, Chien JT, Ho GH, Yang J, Chen BH (2006)Equilibrium and Kinetics Studies on Sorption of Basic Dyes by a Natural Polymer ( γ – Glutamic Acid). Biochem Eng J 31: 204-215.
- Wallace MA (2003) An Evaluation of Copper Biosorption by Brown Seaweed Under Optimized Conditions. Environ Biotechnol 6:174-184.
- Weber WJ (1972) Physico-Chemical Processes for Water Quality Control, John Wiley and Sons Inc., New York, NY.
- Sun XF, Wang SG, Liu XW, Gong WX, Bao N, et al. (2008) Biosorption of Malachite Green from Aqueous Solutions onto Anaerobic Granules: Kinetic and Equilibrium Studies. Bioresour Technol 99: 3475-3483.
- Yavuz O, Altunkaynak Y, Guzel F (2003)Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res 37: 948-952.
- Zhu B, FanT, Zhang D (2008) Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J Hazard Mater 153: 300-308.

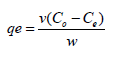 (3)
(3)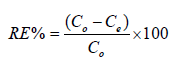 (4)
(4)
 (8)
(8)
 (9)
(9)
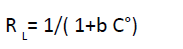 (10)
(10)