Short Communication - (2024) Volume 0, Issue 0
Repurposing Cancer Drugs for Type 1 Diabetes Prevention
Norie Sugitani1* and
Jon D. Piganelli2
1Department of Pediatrics, University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh, Pittsburgh, PA, United States of America
2Department of Endocrinology, Indiana University, Indianapolis, United States of America
*Correspondence:
Norie Sugitani, Department of Pediatrics, University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh,
Pittsburgh, PA,
United States of America,
Email:
Received: 19-Jan-2024, Manuscript No. IPP-24-18933;
Editor assigned: 24-Jan-2024, Pre QC No. IPP-24-18933 (PQ);
Reviewed: 07-Feb-2024, QC No. IPP-24-18933;
Revised: 14-Feb-2024, Manuscript No. IPP-24-18933 (R);
Published:
21-Feb-2024
Description
Type 1 Diabetes (T1D) is an incurable, chronic autoimmune disorder characterized by destruction of insulin- secreting pancreatic β cells by diabetogenic T cells [1-3]. Despite affecting three million Americans, there is currently no preventive measure for this disease [2]. In 2022, Taplizmab became the first and only FDA-approved drug to significantly delay the onset of T1D [4]. However, due to the heterogeneity of T1D and the necessity of antibody infusion in children, the development of additional drugs to delay the onset and ultimately prevent T1D is urgently needed.
Cancer drugs targeting rapid proliferation for T1D prevention
To prevent T1D, it is essential to shield insulin-
producing pancreatic β cells from destruction by self-
antigen activated T cells [1-3]. Similar to many cancer cells, diabetogenic T cells exhibit glycolysis, resembling the Warburg effect, to sustain their rapid proliferation [5- 8]. When naïve T cells are activated to become effector T cells against pancreatic β cells, they go through metabolic switch from oxidative phosphorylation to aerobic glycolysis (Figure 1)
In contrast, terminally differentiated memory T cells uses oxidative phosphorylation [5,6]. Cancer drugs targeting high proliferation rate, stimulation, and glycolysis can potentially target diabetogenic T cells and prevent T1D without minimal effects on naïve or memory T cell population [9-15] ( Figure 1). Utilizing cancer drugs already in clinical trials or FDA-approved provides the advantage of having existing efficacy and safety data in humans.
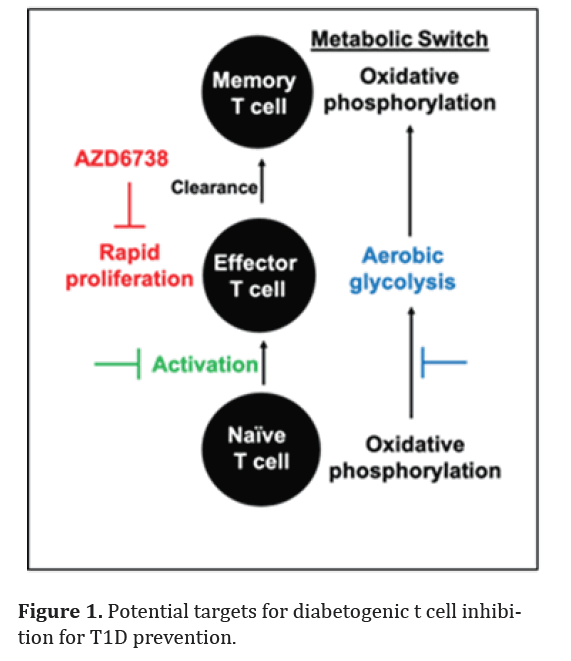
Figure 1: Potential targets for diabetogenic t cell inhibition for T1D prevention.
A cancer drug ceralasertive (AZD6738) prevents T1D in a mouse model
We recently reported a study in Frontiers in Immunology titled “An orally available cancer drug AZD6738 prevents type 1 diabetes”, detailing our efforts to repurpose AZD6738 for T1D prevention [16]. AZD6738, an oral inhibitor of ataxia telangiectasia mutated and Rad3-elated (ATR) kinase, is currently in phases I and II clinical trials for multiple cancers [17-21]. In this study, AZD6738 effectively prevented T1D in an adoptive transfer mouse model by inducing specific cell death in rapidly proliferating diabetogenic T cells [16]. Its mechanism of action mirrors its impact in cancer, involving excess DNA replication origin firing in rapidly proliferating T cells, resulting in DNA damage accumulation, IFNγ production inhibition, and reduced proliferation [16,22]. Importantly, even with an extended treatment period of up to 5 weeks, AZD6738 demonstrated no adverse effects [16]. The oral availability of AZD6738 is advantageous, particularly for patients where antibody infusion is not preferred option.
Future of AZD6738 in T1D prevention
Given that this study is in its early stage, there are many hurdles to pass for AZD6738 to be applied in human T1D prevention. The verification of whether self-
antigen-activated T cells resurge from the naïve T cell pool needs confirmation, in a spontaneous disease model, as the adoptive transfer model utilized in this study lacks endogenous naïve T cells [23,24]. Highly proliferative T cells are susceptible to AZD6738, with effector T cells reported to undergo up to four divisions daily, a rate faster than many activated leukocytes or cancer cells [22,25-29]. However, thorough investigation into potential off-target effects on other cell types is essential prior to human application. A subpopulation of memory T cells crucial for host-defense can proliferate rapidly, potentially making them susceptible to damage by AZD673830. Therefore, designing the treatment schedule will be crucial to maximize diabetogenic T cell inhibition while mitigating the risk of myelosuppression, reported in some cancer clinical trials involving extended treatment periods [30-32].
Other cancer drugs and combination therapy to consider for T1D prevention
Combination therapy is designed to enhance potency by employing multiple agents, enabling the use of lower doses of each, thereby reducing potential toxicity [33-35]. In cancer clinical trials, AZD6738 is frequently combined with other anti-cancer agents [18,36,37]. The specificity of AZD6738 towards highly proliferative cells makes it advantageous for combination with glycolysis inhibitors in T1D prevention. While both agents target diabetogenic T cells, they address different aspects AZD6738 focuses on the high proliferation rate, while glycolysis inhibitors address the transition to aerobic glycolysis metabolism (Figure 1). One potential candidate for combination with a proliferation inhibitor like AZD6738 is the glycolysis inhibitor PFK15 [12]. PFK15 has demonstrated a significant delay in the onset of T1D by inducing terminal exhaustion in diabetogenic T cells in an adoptive transfer mouse model [38]. Combining AZD6738 and PFK15 may permit the use of lower doses for each agent, enhancing their diabetogenic T cell inhibition activity. Given that AZD6738 does not impact T cell activation [16,22], it may also be combined with drugs targeting activation [15]. Besides AZD6738, several FDA-approved or clinically-
trialed DNA replication inhibitors show potential for preventing or delaying the onset of T1D [39-43]. For instance, methotrexate, known for its ability to inhibit T cell proliferation, has been successfully repurposed for treating another autoimmune disorder, rheumatoid arthritis [39]. While further investigation is needed to assess its impact on T cells, imatinib has demonstrated the prevention of pancreatic β cell apoptosis [44].
The exploration of cancer drugs targeting DNA replication for T1D prevention, exemplified by AZD6738, is a promising avenue in pre-clinical studies. Inhibition of T cell proliferation, glycolysis, or activation may collectively contribute to prevent T1D while mitigating off-target effects. The repurposing of existing cancer drugs opens up possibilities for novel therapeutic strategies in the prevention of T1D.
References
- Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010; 464(7293): 1293-1300.
[PMID: 20432533]
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3(1):1-7.
[PMID: 28358037]
- Zhu W, Ye L, Zhang J, Yu P, Wang H, Ye Z, et al. PFK15, a small molecule inhibitor of PFKFB3, induces cell cycle arrest, apoptosis and inhibits invasion in gastric cancer. PloS one. 2016;11(9):e0163768.
[PMID: 27669567]
- Goldman JD, Choi H. Teplizumab: The first treatment to delay the progression of type 1 diabetes. Clinical Diabetes. 2023;41(3):474-476.
[PMID: 37158990]
- Michalek RD, Rathmell JC. The metabolic life and times of a T‐cell. Immunol Rev. 2010;236(1):190-202.
[PMID: 20636818]
- Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 2012;249(1):14-26.
[PMID: 22889212]
- Yu H, Jacquelot N, Belz GT. Metabolic features of innate lymphoid cells. J Exp Med. 2022;219(11):e20221140.
[PMID: 36301303]
- Li H, Zhao A, Li M, Shi L, Han Q, Hou Z. Targeting T-cell metabolism to boost immune checkpoint inhibitor therapy. Front Immunol. 2022;13:1046755.
[PMID: 36569893]
- Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Mol Biol Cell. 2012;23(1):1-6.
[PMID: 22210845]
- Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118(3):312-24.
[PMID: 29123260]
- Onyema OO, Decoster L, Njemini R, Forti LN, Bautmans I, de Waele M, et al. Chemotherapy-induced changes and immunosenescence of CD8+ T-cells in patients with breast cancer. Anticancer Res. 2015;35(3):1481-1489.
[PMID: 25750301]
- Clem BF, O'Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, et al. Targeting 6-Phosphofructo-2-Kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12(8):1461-1470.
[PMID: 23674815]
- Ros S, Schulze A. Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2, 6-bisphosphatases in cancer metabolism. Cancer Metab. 2013;1:8.
[PMID: 24280138]
- Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Investig Drugs. 2008;17(10):1533-1545.
[PMID: 18808312]
- Vincenti F, Luggen M. T cell costimulation: a rational target in the therapeutic armamentarium for autoimmune diseases and transplantation. Annu Rev Med. 2007;58:347-358.
[PMID: 17020493]
- Sugitani N, Mason HR, Campfield BT, Piganelli JD. An orally available cancer drug Azd6738 prevents type 1 diabetes. Front Immunol. 2023;14: 1290058.
[PMID: 38164129]
- Foote KM, Nissink JW, McGuire T, Turner P, Guichard S, Yates JW, et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and rad3 related (ATR) kinase with application as an anticancer agent. J Med Chem. 2018; 61: 9889-9907.
[PMID: 30346772]
- Dillon MT, Boylan Z, Smith D, Guevara J, Mohammed K, Peckitt C, et al. PATRIOT: A phase i study to assess the tolerability, safety and biological effects of a specific ataxia telangiectasia and rad3-related (ATR) inhibitor (AZD6738) as a single agent and in combination with palliative radiation therapy in patients with solid tumors. Clin Transl Radiat Oncol. 2018;12:16-20.
[PMID: 30073210]
- Lecona E, Fernandez-Capetillo O. Targeting ATR in cancer. Nat Rev Cancer. 2018;18(9):586-595.
[PMID: 29899559]
- Bradbury A, Hall S, Curtin N, Drew Y. Targeting ATR as cancer therapy: A new era for synthetic lethality and synergistic combinations? Pharmacol Ther. 2020;207:107450.
[PMID: 31836456]
- Brandsma I, Fleuren ED, Williamson CT, Lord CJ. Directing the use of DDR Kinase Inhibitors in Cancer Treatment. Expert Opin Investig Drugs. 2017;26(12):1341-1355.
[PMID: 28984489]
- Sugitani N, Vendetti FP, Cipriano AJ, Pandya P, Deppas JJ, Moiseeva TN, et al. Thymidine rescues ATR kinase inhibitor-induced deoxyuridine contamination in genomic DNA, cell death, and interferon-alpha/beta expression. Cell rep. 2022;40(12).
[PMID: 36130512]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptiveimmunologic function in NOD/Ltsz-scid Mice. J Immunol. 1995;154(1):180-191.
[PMID: 7995938]
- Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice: Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42(1):44-55.
[PMID: 8093606]
- Yoon H, Kim TS, Braciale TJ. The cell cycle time of CD8+ T cells responding in vivo is controlled by the type of antigenic stimulus. PloS one. 2010;5(11):e15423.
[PMID: 8093606]
- Lee WT, Pasos G, Cecchini L, Mittler JN. Continued antigen stimulation is not required during CD4+ T cell clonal expansion. J Immunol. 2002;168(4):1682-1689.
[PMID: 11823497]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-1535.
[PMID: 15001782]
- Amanna, IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010; 236:125-138.
[PMID: 20636813]
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006; 211: 81-92.
[PMID: 16824119]
- Macallan DC, Wallace D, Zhang Y, de Lara C, Worth AT, Ghattas H, et al. Rapid turnover of effector memory CD4+ T cells in healthy humans. J Exp Med. 2004; 200: 255-260.
[PMID: 15249595]
- Yap TA, Krebs MG, Postel-Vinay S, El-Khouiery A, Soria JC, Lopez J, et al. Ceralasertib (AZD6738), an oral ATR kinase inhibitor, in combination with carboplatin in patients with advanced solid tumors: a phase i study. Clin Cancer Res. 2021; 27:5213-5224.
[PMID: 34301752]
- Kim ST. Phase I study of ceralasertib (AZD6738), a novel DNA damage repair agent, in combination with weekly paclitaxel in refractory cancer. Clin Cancer Res. 2021;27:4700-4709.
- Bayat Mokhtari R. et al. Combination therapy in combating cancer. Oncotarget. 2017; 8:38022-38043.
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328-337.
- Hu M. Synergistic combination chemotherapy of camptothecin and floxuridine through self- assembly of amphiphilic drug-drug conjugate. Bioconjug Chem. 2015;26:2497-2506.
[PMID: 26497258]
- Ring A. Olaparib and Ceralasertib (AZD6738) in Patients with Triple-Negative Advanced Breast Cancer: Results from Cohort E of the plasmaMATCH Trial (CRUK/15/010). Clin Cancer Res. 2023;29:4751-4759.
- Kwon M. Phase II Study of Ceralasertib (AZD6738) in Combination with Durvalumab in Patients with Advanced Gastric Cancer. J Immunother Cancer. 2022;10.
- Martins CP. Glycolysis Inhibition Induces Functional and Metabolic Exhaustion of CD4+ T Cells in Type 1 Diabetes. Front Immunol. 2021; 12: 669456.
[PMID: 34163475]
- Cronstein BN. Low-dose methotrexate: A Mainstay in the Treatment of Rheumatoid Arthritis. Pharmacol Rev. 2005; 57:163-172.
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of Action and Clinical Strategies. Nat Rev Cancer. 2003;3:330-338.
- Druker BJ. Effects of a Selective Inhibitor of the Abl Tyrosine Kinase on the growth of Bcr-Abl Positive Cells. Nat Med.1996; 2:561-566.
- Rosenberg B, Vancamp L, Krigas T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature. 1965;205:698-699.
- Gewirtz D. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem Pharmacol. 1999;57(7):727-741.
[PMID: 10075079]
- Kutpruek S, Suksri K, Maneethorn P, Semprasert N, Yenchitsomanus PT, Kooptiwut S. Imatinib Prevents Dexamethasone‐Induced Pancreatic β‐Cell Apoptosis via Decreased Trail And Dr5. J Cell Biochem. 2023;124(9):1309-23.
[PMID: 37555250]
Citation: Sugitani N, Piganelli JD. Repurposing Cancer Drugs for Type 1 Diabetes Prevention. JOP. J Pancreas. (2024) 24:13-16.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
