Research Article - (2023) Volume 9, Issue 1
Repurposing of Favipiravir for the Treatment COVID-19: A Meta-analysis
Velichka Pavlova1,
Katya Uzunova1,
Elena Filipova1,
Krassimir Kalinov2 and
Toni Vekov3
1Department of Science, Tchaikapharma High Quality Medicines, Bulgaria
2Department of Informatics, Medistat Ltd, Bulgaria
3Department of Pharmacy, Medical University, Bulgaria
Received: 02-Jan-2023, Manuscript No. IPJIDT-23-15376;
Editor assigned: 04-Jan-2023, Pre QC No. IPJIDT-23-15376 (PQ);
Reviewed: 18-Jan-2023, QC No. IPJIDT-23-15376;
Revised: 23-Jan-2023, Manuscript No. IPJIDT-23-15376 (R);
Published:
30-Jan-2023, DOI: 10.36648/2472-1093-9.1.05
Abstract
Background: The outbreak of coronavirus disease 2019 (COVID-19) originating from Wuhan, Hubei Province, China at the end of 2019 led to dramatic changes in the healthcare and socioeconomic sectors across the globe. The aim of this meta-analysis was to assess whether favipiravir is a safe and effective option for treatment of COVID-19 patients compared with standard of care (SOC) and/or other applied medicines.
Methods: Data bases were searched up to 31st May 2021 for studies that compare the efficacy and safety of favipiravir and SOC or other relevant therapy in COVID-19 patients. Search results were assessed for relevance on the basis of the following inclusion criteria and relevant results were subjected to a quality estimation using the EPHPP Quality assessment tool.
Results: A total of 10 articles with hospitalized patients and outpatients (n=1016) met our inclusion criteria. Pooled RR 1.24 (95% CI 1.08-1.43, n=5) showed clinical improvement by Day 7 and Day 14 (pooled RR 1.18 (95% CI 1.01-1.37, n=5) and favipiravir was associated with 24% and 18% better outcome compared to other treatment, respectively. Viral clearance by Day 7 and Day 14 with favipiravir was comparable to other treatments (RR 1.1; 95% CI 0.92-1.35, n=5) and (RR 1.07, 95% CI 0.88-1.29, n=5). Safety profile of favipiravir was comparable to that of other treatments (RR 1.21; 95% CI 0.88-1.67) and SEA including death were comparable between treatments (RR 0.64, 95% CI 0.15-2.68, n=4 studies). No correlation between incidence of SEA and treatment option was identified.
Conclusion: There is a significant difference in the clinical improvement detected on Days 7 and 14 in favour of favipiravir. Viral clearance at Days 7 and 14 is comparable between treatments with neither being associated with significantly better outcomes. The safety profiles of favipiravir and SOC regarding SAE show no statistically significant differences.
Keywords
Favipiravir, COVID-19, Meta-analysis, Efficacy, Safety
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) originating
from Wuhan, Hubei Province, China at the end of 2019 led to
dramatic changes in the healthcare and socioeconomic sectors
across the globe. The virus was named by World Health Organization
(WHO) as the 2019 novel Coronavirus and was renamed on
11th Feb 2020 as SARS-CoV-2 [1]. According to the Johns Hopkins
Coronavirus Resource Center until now over 418,235,000 confirmed
Global COVID-19 cases and over 5,850,000 deaths have
been reported [2]. SARS-CoV-2 belongs to a family of Coronaviruses
[3]. They are single-stranded, positive-sense, RNA containing,
and enveloped viruses with a genome size between 27 and
34 kilobases that is comparatively larger than other RNA viruses
[3-5]. The investigations showed that SARS-CoV-2 have 75%-80%
identical genome sequence as SARS-CoV [6,7].
There were two global epidemics of atypical pneumonia SARSCoV
and MERS-CoV, respectively in 2002 and 2012 and later
MERS-CoV reappearing in South Korea in 2015 [8-10]. Despite
the lower mortality rate of SARS-CoV-2 infection compared to
MERS-CoV (9.5%) and SARS-CoV (34.4%), the COVID-19 pandemic
has raised a significant concern [11].
The observed serious, life-threatening manifestations and complications
after infection with the virus were caused by the severe
respiratory syndrome, related with diffuse alveolar damage
and severe lung injury [12]. It was suggested that different
systems might be involved, including respiratory (cough, shortness
of breath, rhinorrhea, sore throat, hemoptysis, and chest
pain), musculoskeletal (muscle ache), gastrointestinal (Diarrhea,
abdominal pain and vomiting), olfactory (hyposmia, anosmia or
complete loss of olfactory functions), ophthalmic (conjunctivitis,
retinitis), dermatological (erythematous rash, chickenpox-like
vesicles), cardiovascular (arrhythmias), rheumatological (arthralgia)
and neurologic (headache and confusion) [13-15]. COVID-19
has incubation period between 5-6 days that can be extended up
to 14 days [16]. In pediatric patients when compared to adults,
the incubation period is a little bit longer up to 14 days [17].
Due to wide prevalence nature of SARS-CoV-2, its mortality rate,
and its limited treatment options new therapeutic alternatives
need to be provided. One possibility is to repurpose already
existing drugs, which would provide beneficial and immediate
effects on COVID-19 patients. Globally, the clinical researchers
were testing many existing drugs, such as hydroxychloroquine/
chloroquine approved to treat Malaria; antiretrovirals lopinavir/
ritonavir and darumavir/ritonavir; the serine protease inhibitors
camostat mesylate and nafamostat mesylate; anti-parasitic drug
ivermectin; drugs that interfere cytokine activities as tocilizumab,
sarilumab, and IL-1 receptor antagonist anakinra; anti-inflammatory
drugs, including corticosteroids as dexmethazone;
anticancer drugs as dasatinib, imatinib and nilotinib; remdesevir
originally approved to treat HIV and other nucleoside analogues:
Ribavirin, galidesivir and favipiravir ect [18,19].
On February 15, 2020 in China, favipiravir was approved as treatment
option for this life threatening infection [20]. Favipiravir,
also known as a T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide),
initially was developed in 2002 at Research Laboratories of
Toyama Chemical Co., Ltd, Japan. This is a prodrug of a purine nucleic
acid analog, that is phosphoribosylated by cellular enzymes
to its active form favipiravir-ribofuranosyl-5’-triphosphate [21].
The mechanism of action include inhibition of RNA dependent
RNA polymerase (RdRP), that is needed for RNA viral replication
within infected cells, acting as a purine analog and is incorporated
instead of guanine and adenine [22].
Favipiravir has a wide range of antiviral effects in vitro and in vivo and this can be explained by the fact that catalytic domain of
RdRP is evolutionary conserved in various RNA viruses. Favipiravir
inhibits 53 types of influenza viruses. The range includes
influenza A (H1N1, H2N2, H3N2, H4N2, H7N2, H5N1 and other
strains), influenza B [23,24] and many other RNA viruses (Arenaviruses,
Phleboviruses, Hantaviruses, Flaviviruses, Enteroviruses;
an alphavirus, a paramyxovirus, respiratory syncytial virus, and noroviruses) [25-27]. However, favipiravir showed weak activity
against non-influenza virus RNA viruses and it had no activity to
DNA viruses. An in vitro study showed that favipiravir has potent inhibitory activity against influenza A, B, and C viruses. The IC50s
ranged from 0.013 μg/ml to 0.48 μg/ml for the influenza A viruses,
from 0.039 μg/ml to 0.089 μg/ml for the influenza B viruses,
and from 0.030 μg/ml to 0.057 μg/ml for the influenza C
viruses [28]. Moreover, several studies showed its effectiveness
against Ebola virus [29]. Favipiravir suppressed replication of Ebola
virus in cell culture by 4 log 10 units with an IC90 of 110 μM
[30]. In vitro experiments with favipiravir demonstrated that half
maximal effective concentration (EC50)-61.88 μM/L of favipiravir,
half-maximal cytotoxic concentration (CC50)>400 μmol. L-1,
selectivity index (SI)>6.46 effectively inhibits SARS-CoV-2 in Vero
E6 cells [31].
Furthermore, in mice lacking type I interferon-alfa/beta receptor
(IFNAR-/-) was established that at Day 6 post infection (corresponds
to 2-4 days before the time of death in control animals)
favipiravir induced rapid virus clearance, reduced viremia, ameliorated
clinical and biochemical signs of disease, and prevented
a lethal outcome in 100% of the animals [30]. Furuta et al., 2002
observed in mice, which were infected with influenza virus A/
PR/8/34, that administration of favipiravir at 100 mg/kg of body
weight/day (four times a day) for 5 days was associated with significant
reduction in the mean pulmonary virus yields and the
rate of mortality.
The results from clinical trials conducted with favipiravir in
COVID-19 patients are conflicting and non-conclusive. Therefore,
we tried to summarize the existing data to boost the information
about efficacy and safety profile of favipiravir in patients with
COVID-19. The aim of the present meta-analysis was to establish
with an acceptable level of confidence the improvement and
tolerability rates in patients with COVID-19 after favipiravir treatment
compared to standard of care and/or other drugs.
Methods
Data Sources and Search Strategy
We searched the following databases from the beginning of
2020 to the end of May 2021, for relevant studies: MEDLINE,
SCOPUS, PsyInfo, eLIBRARY.ru, as well as the clinical trial registries
for unpublished data (https://www.clinicaltrialsregister.eu/;
https://clinicaltrials.gov/; www.chictr.org) and preprint databases
MedRxiv and Research Square. The following keywords and
various combinations were used in the search: “Coronavirus”
OR “COVID-19” OR “SARS-CoV-2” AND “Favipiravir” OR “Avigan”
AND “clinical trial” AND “controlled” AND “randomi*” AND “double
blind.”
Full-text articles and abstracts published in English and Cyrillic
were checked for relevance to the topic and were assessed.
Eligibility Criteria and Quality Assessment
Search results were assessed for relevance on the basis of the
following inclusion criteria:
• Type of study/trial-epidemiological, controlled and randomized;
• Studies providing information for the investigation of clinical
improvement, including assessment of symptoms and radiological
results and/or time to negative PCR (information
about viral clearance) and/or worsening of clinical symptoms or necessity of supplemental oxygen therapy and/or
safety of treatments applied;
• Types of subjects representatives of the whole population,
specific stratum;
• Patients with proven SARS-CoV-2 infection;
• Access to source data;
• Eligibility for statistical analysis. Studies that correspond to
the inclusion criteria were subjected to a quality estimation
using the EPHPP Quality assessment tool (Table S1). Sources
were excluded if they represented trials in which the principle
arm reported other outcomes different from changes
in clinical condition (improvement or worsening), time to
negative PCR test and safety investigation; other conditions
apart from COVID-19.
This tool includes assessment of different characteristics like selection
bias, study design, blinding, data collection method, confounders,
and drop outs in order to help raters form an opinion of quality based upon information contained in the study. Studies
that correspond to the aforementioned inclusion criteria are
subjected to quality estimation and general ratings are taken into
account when results from the study are interpreted.
Data Extraction
All available studies were carefully reviewed and assessed for
relevance according to the predefined inclusion criteria. Figure
1 represents the process of studies selection in order to determine
their eligibility for inclusion in the analysis. After removing
redundant articles and abstracts then only full-text articles
were investigated. Two reviewers independently extracted data.
Extracted data includes the following items: Author’s name, type
of study, year of publication, sample size, target population, type
of intervention, dose of intervention, control group, primary and
secondary outcomes, follow-up and/or treatment duration (Table
1). Outcome variables were extracted and are represented in
different tables (Tables 2 and 3). The studies with insufficient or
incomplete data were not included. Any potential disagreements were resolved through discussion among the authors.
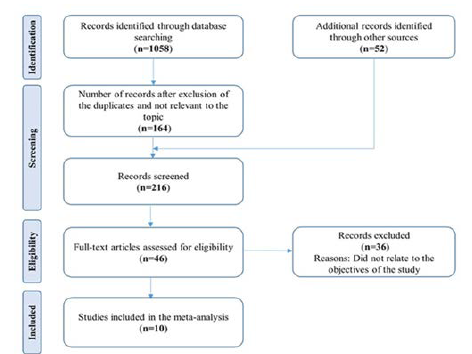
Figure 1: Search process and study flow diagram
| Study: First author (year) |
Type of study |
Sample Size*Test/
Reference |
Target population |
Market or INN Name |
FVP Dose (daily) |
Control group |
Primary/secondary endpoints |
Follow-up/treatment duration (days) |
Efficacy |
Safety |
Balykova et al. (2020) |
open-label, randomized, multicenter comparative study |
17/22 |
Hospitalized with moderate COVID-19 pneumonia |
replivir |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2-14 |
SOC |
Clinical recovery/Viral clearance |
TEAE |
14/15 |
Chen et al. (2020) |
prospective, randomized, controlled, open-label multicenter trial |
116/120 |
Inpatients, moderate/severe |
FVP |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2-10 |
Umifenovir (Arbidol+SOC |
Clinical recovery |
TEAE |
17-Oct |
critical COVID19 pneumonia |
Cai et al. (2020) |
open-label, nonrandomized, controlled
study |
35/45 |
Inpatients, moderate COVID19 simptoms |
FVP+INFalfa1b |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2-14 |
lopinavir/ritonavir+INFalfa1b |
Clinical improvement/Viral clearance |
TEAE |
14/14 |
Dabbous et al. (2020) |
randomized-controlled open-label interventional clinical trial |
50/50 |
Inpatients, mild/moderate COVID19 symptoms |
FVP+enoxaparin |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2-10 |
Hydroxychloro |
Viral clearance |
TEAE |
30-Oct |
quine/oseltamivir |
+enoxaparin |
Dabbous et al. (2021) |
multicenter randomized controlled study |
44/48 |
Inpatients, mild/moderate COVID19 symptoms |
FVP+SOC |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2-10 |
Chloroquine+SOC |
Clinical improvement/Mortality |
TEAE |
-/10 |
Ivashchenko et al. (2021) |
adaptive, multicenter, open label, randomized,
Phase II/III clinical trial |
20/20 |
Inpatients, moderate COVID19 pneumonia |
Avifavir |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2-14 |
SOC |
Clinical improvement/Viral clearance |
TEAE |
29/14 |
|
20/20 |
Inpatients, moderate COVID19 pneumonia |
Avifavir |
1800 mg b.i.d. day 1/800 mg b.i.d. day 2-14 |
SOC |
Clinical improvement/Viral clearance |
TEAE |
29/14 |
Lou et al.(2021) |
exploratory single center, open-label, randomized, controlled trial |
##### |
Inpatients, |
FVP+SOC |
1600 or 2200 mg day 1/600 mg t.i.d. day 2-14 |
SOC |
Clinical improvement/Viral clearance |
TEAE |
-/14 |
COVID-19 patients |
Baloxavir+SOC |
Ruzhentsova et al. (2020) |
open-labeled, randomized, active-controlled multicenter trial |
112/56 |
Outpatients and in patients with mild to moderate COVID-19 |
FVP |
1800 mg b.i.d. day 1/800 mg b.i.d. day 2-10 |
SOC |
Clinical improvement/Viral clearance |
TEAE |
28-Oct |
Udwadia et al. (2021) |
open-label, randomized, parallel-arm, multicenter trial |
72/75 |
Inpatients, mild/moderate COVID-19 |
FVP+SOC |
1800 mg b.i.d. day 1/800 mg b.i.d. day 2-14 |
SOC |
Viral clearance/Clinical cure |
TEAE |
28/14 |
Zhao et al. (2021) |
multicenter, open-label, randomized controlled trial |
36/19 |
Re-positive outpatients, mild/moderate |
FVP+SOC |
1600 mg b.i.d. day 1/600 mg b.i.d. day 2 to 7-14 |
SOC |
Viral clearance |
TEAE |
##### |
Note: FVP-Favipiravir; SOC-Standard of care; TEAE-treatment emergent adverse event
*The sample size includes only patients who participated in the comparative analysis (ITT population)
Table 1: Summary of characteristics of the included studies
| Study: First author (year) |
Outcome measures |
| Clinical improvement |
Virus clearance |
| Day 4-7 |
Day 10-14 |
Day 4-7 |
Day 10-14 |
Day 28 |
| FVP |
Control |
FVP |
Control |
FVP |
Control |
FVP |
Control |
FVP |
Control |
| N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
| Cai et al. (2020) |
8/27 |
8/37 |
32/3 |
28/17 |
|
|
|
|
|
|
| Chen et al. (2020) |
71/45 |
62/58 |
|
|
|
|
|
|
|
|
| Dabbous et al. (2020) |
|
|
|
|
24/26 |
28/22 |
48/2 |
45/5 |
|
|
| Ivashchenko et al. (2020) |
|
|
36/4 |
16/4 |
25/15 |
6/14 |
37/3 |
16/4 |
|
|
| Lou et al. (2021) |
2/7 |
1/9 |
5/4 |
5/5 |
4/5 |
5/5 |
7/2 |
10/0 |
|
|
| Ruzhentsova et al. (2020) |
59/53 |
20/36 |
93/19 |
37/19 |
91/21 |
38/18 |
|
|
|
|
| Udwadia et al* (2021) |
45/8 |
34/15 |
48/5 |
44/5 |
45/27 |
44/31 |
66/6 |
60/15 |
|
|
| Zhao et al. (2021) |
|
|
|
|
|
|
|
|
29/7 |
10/9 |
Note: N1=cases, N2=non-cases
*Clinical evaluation on days 7 and 14 had 53 patients with FVP and 49 with SOC.
Table 2: Reported outcome measures for clinical improvement and viral clearance.
| Study: First author (year) |
Adverse Events |
Serious Adverse Events, including death |
| FVP |
Control |
FVP |
Control |
| N1/N2 |
N1/N2 |
N1/N2 |
N1/N2 |
| Balykova et al. (2020) |
11/6 |
16/6 |
- |
- |
| Cai et al. (2020) |
4/31 |
25/20 |
- |
- |
| Chen et al. (2020) |
37/79 |
28/92 |
- |
- |
| Dabbous et al. (2020) |
4/46 |
21/29 |
0/50 |
1/49 |
| Dabbous et al. (2021) |
14/30 |
10/38 |
1/43 |
2/46 |
| Lou et al. (2021) |
8/1 |
9/1 |
- |
- |
| Ruzhentsova et al. (2020) |
80/28 |
33/22 |
2/106 |
0/55 |
| Udwadia et al. (2021) |
26/47 |
6/69 |
0/72 |
1/74 |
| Zhao et al. (2020) |
12/24 |
7/12 |
- |
- |
Note: N1=cases, N2=non-cases
Table 3: Reported AE and SAE including death.
Statistical Analysis
The risk ratio (RR) for efficacy and safety variables with 95% confidence
intervals (CIs) was obtained from each study. Due to the
significant heterogeneity of the individual studies, we chose the
random-effects method as the primary analysis and forest plots
were constructed. I2 statistics and Cochran test were used to assess
the heterogeneity of the included studies where p values of
less than 0.10 were used as an indication of the presence of heterogeneity.
For all analyses, significance levels were two-tailed,
and p<0.05 was considered significant. The value of I2 ranges
from 0% to 100% and I2 <50% indicated that the heterogeneity
of included studies was acceptable.
The sensitivity analyses was carried out by consequently subtracting
each study from the analysis set and calculating the
pooled prevalence and I2 of the remaining studies, in order to
identify studies that may significantly affect the pooled prevalence
and heterogeneity, respectively. Funnel plots were used to
identify and evaluate publication bias.
All analyses were performed using the module MetaXL (add-ins
on Microsoft Excel).
Results
Description of Search
We identified a total of 1058 records after searching the databases
and through other sources. The number of screened records
was 216 after the removal of duplicates or unrelated to the topic.
Only 46 full-text articles were assessed and 36 from them were
excluded on the bases of predefined inclusion criteria. Finally, 10
studies were included in our meta-analysis. The complete study
selection process is shown in Figure 1.
Characteristics of the Included Studies
Ten studies met the inclusion criteria and were subject to analysis.
The included studies were published between 2020 and
2021, and were registered in clinical trial registries. Only one
study was nonrandomized. Results in Cyrillic were not included.
Summarized characteristics of target population are given in Table
1. In the present meta-analysis we evaluated incidence of improvement
or deterioration among favipiravir group in comparison
to SOC or other antivirals at day 4-7 and at day 10-14 (Table
1) as well as viral clearance at day 4-7, at day 10-14 and at day 28
(Table 2) and adverse events or serious adverse events including
death (Table 3) identified during use of all treatments.
The minimum follow-up time in all included studies was 14 days,
and the maximum was 30 days. The dose of favipiravir in each
study was different but generally matches the standard dose for
treating influenza infection. All studies included patients with
proven COVID-19. The target population was hospitalized patients
in [32-38]; one study included outpatients and in patients
with mild to moderate COVID-19 [39] and in one study the target
population was re-positive outpatients [40].
Outcomes of the Meta-Analysis
Clinical improvement: Five studies assessed clinical improvement
at 4-7 days and five studies at 10-14 days (Figures 2 and 3) respectively
[33-39].
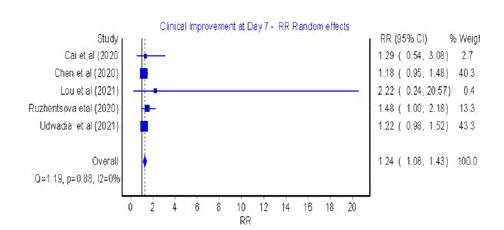
Figure 2: Forest plot: Clinical improvement at Day 7
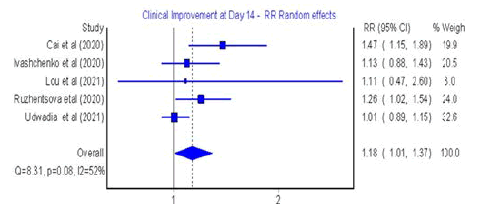
Figure 3: Forest plot: Clinical improvement at day 14 of favipiravir treatment
The analysis showed that significant clinical improvement was
achieved in the favipiravir group versus the control group at Day
7 (RR=1.24; 95% CI: 1.08-1.43; Q=1; p=0.88; I2=0%) (Figure 2).
The presented results were homogeneous with symmetrical
distribution (Figure S1). Furthermore, in 14 days, the clinical improvement
with favipiravir was 18% higher than with other treatments,
but this result was not statistically significant (RR=1.18;
95% CI: 1.01-1.37; Q=8.31; p=0.08; I2=52%, with evidence of low
heterogeneity and symmetrical distribution (Figure S2)
Viral Clearance
Among the included studies, five studies assessed viral clearance
after 4-7 days (Figure 4), five after Day 10 of treatment [34,36-40]
(Figure 5).
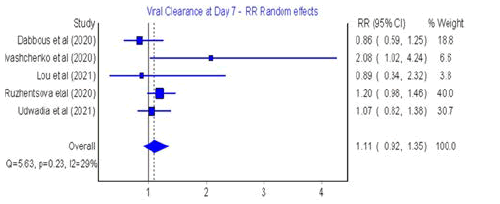
Figure 4: Forest plot: Viral clearance after Days 4-7 of favipiravir treatment.
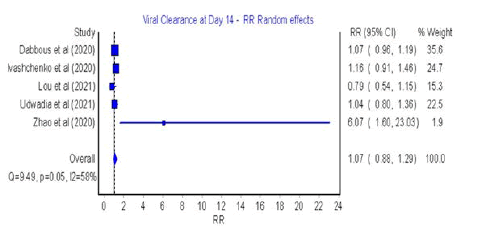
Figure 5: Forest plot: Viral clearance after day 10
The meta-analysis of risk ratios (RR) for favipiravir compared
with SOC or other antivirals showed that there was no significant difference at 7 days and 10 days post treatment (RR=1.11; 95%
CI: 0.92-1.35; Q=5.63; p=0.23; I2=29% for 4-7 days and RR=1.07;
95% CI: 0.88-1.29; Q=9.49; p=0.05; I2=58% for 10 days post treatment).
(Figures S3 and S4) in Supplementary materials shows asymmetric
distribution of the results with low insignificant heterogeneity
(Q=5.63, p>0.05, I2=29% for 4-7 days; Q=9.49, p=0.05, I2=58%
for 10-14 days post treatment).
Adverse Events, Serious Adverse Events including
Mortality
All studies included in the meta-analysis reported adverse events
and among them only four contained information about serious
adverse events including death [34,36,39,41]. Favipiravir treatment
did not lead to more adverse outcomes in comparison to
control group (RR=1.21; 95% CI=0.88–1.67; Q=35.49; p=0.00;
I2=77%), as presented in Figure 6, but it was accompanied by a
high heterogeneity across the included studies (p<0.05) and low
asymmetry in the results (Figure S5).
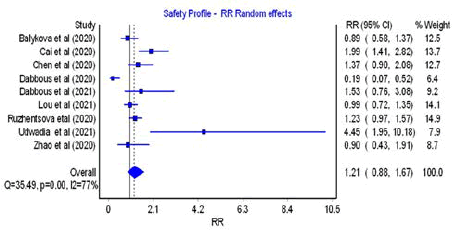
Figure 6: Forest plot: Safety profile
Based on the meta-analysis, the observed serious advese events
including death in the favipiravir group was approximately 36%
less than the control group, but this finding was not statistically
significant (RR=0.64; 95% CI=0.15-2.68; Q=1.14; p=0.77; I2=0%)
with no evidence of inter study heterogeneity and low asymmetric
distribution of the results (Figure S6).
Sensitivity Analysis
The results from sensitivity analysis (Tables S2-S7) suggested that
the sequential exclusion of individual studies did not lead to large
variation in final risk ratios, although there were differences in
assessment of weight. The lack of substantial changes in RR suggests
consistency in findings and is a tentative confirmation of
the possible prevalence for favipiravir compared to the alternatives.
Discussion
Huge amount of efforts would be spent for the development of
a new appropriate and effective medicine to treat COVID-19.
Meanwhile patients could benefit from a number of approved
and already marketed antiviral drugs that need to be repurposed
for the current pandemic. A good candidate for that purpose
could be is favipiravir. Its first indication is treatment of flu and
other viral infections. The drug was firstly used as treatment option
for COVID-19 in China, and at the present time favipiravir is
approved for use in, Japan, Russia, Ukraine, Uzbekistan, Moldova,
Kyrgyzstan, and Saudi Arabia, UAE, Turkey and others. Currently
there are more than 30 clinical trials assessing the efficacy
of favipiravir against COVID-19 worldwide according to https://
clinicaltrials.gov/ [42] (excluding bioequivalence/bioavailability
studies).
There is no uniform way to assess clinical efficacy and safety of
Favipiravir compared to SOC or other therapies in patients with
COVID-19. Our meta-analysis included 10 studies with a total of
1016 patients. Clinical improvement based on the evaluation of
symptoms and radiology results, negative RT-PCR and progression/
worsening of clinical symptoms or need of mechanical ventilation
were used to measure clinical efficacy.
Safety was assessed by comparative analysis of the number of
adverse events and/or adverse reactions, as well as measurement
of the tolerability of the drug.
In our analysis we investigated the efficacy and safety of favipiravir
in the published literature. We have compared favipiravir with
standard of care (SOC) control or other antiviral agent/combinations.
The obtained results from our study showed 24% higher
clinical improvement with favipiravir in 7 days compared to other
treatments and vary between 7% and 44%. On the other hand,
in 14 days, the clinical improvement with Favipiravir was 18%
higher than with other treatments and varies between 1% and
37%. Viral clearance up to 14 days in patients taking favipiravir
was comparable to that those receiving other drugs. The difference
in viral clearance between favipiravir and reference therapy
was between days 4-7 of treatment (Figure 4). The tendency was
for higher viral clearance by favipiravir, but in order to support
that hypothesis, quantitative measurement rather than RT-PCR
testing should probably be used. Additionally, the safety profile
of favipiravir and that of the reference treatment regarding serious
adverse events and reactions did not differ. The overall risk
assessment was (RR=1.10, 95% CI=(0.82; 1.48) and it was not
statistically significant. It must be noted that the assessment of
AEs was based on different methodology in the different studies
included and it requires careful interpretation. According to our
results, the observed serious adverse events including death in
the favipiravir group was approximately 36% less than the control
group, but this finding is not statistically significant (RR=0.64;
95% CI=0.15-2.68; Q=1.14; p=0.77; I2=0%) (Figure 7). Respectively,
no common relationship between SAEs including death and
favipiravir treatment could be derived.
Clinical improvement and viral clearence were assessed in the
work of Hassanipour et al. There was a significant clinical improvement
in the Favipiravir treatment group after seven days
of drug intake (RR=1.24, 95% CI: 1.09-1.41; P=0.001, I2=0.0%. P=0.939). When compared to 14 days of intervention a non-statistically
significant clinical improvement was observed (RR=1.10,
95% CI: 0.97-1.25; P=0.108, I2=34.5% and P=0.177). Viral clearance
was not statistically significant for days 7-10 and 14, but was
more pronounced for the last day of clinical observation. In the
analysis it was observed that favipiravir group needed 7% less
supplemental oxygen therapy compared to control group but the
finding was not-statistically significant (RR=0.93, 95% CI: 0.67–
1.28; P=0.664, I2=0.0%, P=0.950). Authors reported only mild to
moderate adverse events in both treatment and control group
and mortality rate of 30% less for the favipiravir group which did
not reach statistical significance. All in all, they concluded that
T-705 was administered relatively late and thus its efficacy was
low in the clinical setting [42,43].
Another meta-analysis also examined viral clearance and clinical
improvement as the primary outcomes against COVID-19. Patients
treated with favipiravir had better viral clearance at day 7
after treatment (OR=2.49, 95% CI=1.19-5.22) compared to comparator
group. By day 14 no difference in viral clearance between
the two groups was observed (OR=2.19, 95% CI=0.69–6.95).
Clinical improvement was significantly higher in the favipiravir
group on both day 7 and day 14 compared to comparator group
but prevalence was seen at day 14 (OR=3.03, 95% CI=1.17-7.80).
Conclusion was made that favipiravir caused viral clearance by
day 7 along with clinical improvement within 14 days. Thus, favipiravir
proved to be a reliable option for the treatment of mild to
moderate COVID-19 disease. The early administration of the drug
at the higher end of the dosing range could be an important step
for the treatment of mild or asymptomatic COVID-19 [44].
Quite the contrary is described in the work of Özlüşen B et al.
They claimed that in some countries (Turkey) favipiravir is administered
early in the disease course though with a lack of significant
effect. The effect of favipiravir on fatality rate and mechanical
ventilation in COVID-19 patients was also studied. A total of
12 studies were included in their analysis. Authors did not identify
any superiority of favipiravir over SOC or other antiviral medicines
up to 14 days of treatment. In terms of mechanical ventilation
significant heterogeneity was observed due to high risk of
bias in the included studies. Additionally, it was discussed that
viral clearance and viral load were not appropriate measures to
follow disease progression [45]. Moreover, clinical improvement
was not included in their analysis since clinical improvement differed
between studies and could lead to the notion of subjectivity,
which is contrary to our results.
Another systematic review suggested the effect of three antiviral
drugs, namely remdesivir, favipiravir and lopinavir/ritonavir on COVID-19. When favipiravir was combined with other supportive
therapy (tocilizumab) or given as monotherapy it had beneficial
role on clinical recovery of patients but no significant effect was
noted when compared to control treatment group. Authors believe
it was not appropriate to recommend antiviral drugs to be
used in clinical setting based on the conflicting results from clinical
trials [46].
Efficacy and safety of favipiravir were analysed in the meta-analysis
of Shrestha et al. A significant clinical improvement was noted
on day 14 of drug administration compared to control (RR 1.29,
1.08-1.54). Viral clearance (day 14: RR 1.06, 95% CI 0.84-1.33)
as well as non-invasive ventilation or oxygen requirement (OR
0.76, 95% CI 0.42-1.39), and adverse effects (OR 0.69, 0.13-3.57)
did not show statistical significance when the two groups were
compared. Lastly, it was stated that statistical significance could
be reached for parameters clinical improvement and radiological
improvement and juditial use of favipiravir should be supported
[47].
Additionally, another meta-analysis evaluated the clinical improvement
among COVID-19 patients. Observation was of marginal
beneficial effect that was seen in the favipiravir arm in
overall clinical improvement comparison to SOC/control, i.e., (4
studies, log OR [95% CI] (−0.19 [−0.51, 0.13]). For days 7-10 and
10-14 treatment with favipiravir was comparable to the SOC/control
arm: For day 7-10 (3 studies, OR [95% CI] 1.63 [1.07, 2.48])
and for clinical improvement on day 10-14 (3 studies, OR [95% CI]
1.37 [0.24, 7.82]). Viral negativity after favipiravir treatmrent was
associated with the lower odds as compared to the standard of
care (SOC)/control treatment group (4 studies, OR [95% CI] 1.91
[0.91, 4.01]) [48].
Major guidelines on the treatment of COVID-19 do not recommend
the use of favipiravir because of insufficient and uncertain
evidence for its use. Neither Guidelines of NIH nor WHO recommend
favipiravir for the treatment of COVID-19. Same applies
to Japanese guidelines and Australian guidelines. The Philippine
COVID-19 Living Clinical Practice Guidelines recommend for the
use of favipiravir only in the context of clinical trials. In Belgium
the drug is currently unavailable for treatment outside of clinical
trials. Guidelines of the UAE include favipiravir as treatment option
for confirmed COVID-19 cases at a dose of 1600 mg PO BID ×
2 doses then 600 mg PO BID (total 5 days) among other drugs. In
cases with pneumonia favipiravir could be combined with chloroquine/
hydroxychloroquine and camostat. It could also be given
in combinations with other drugs to critically ill patients for 10
days. Dose might need to be adjusted based on clinical scenario
[49-55].
Favipiravir is believed to be a relatively safe drug. Pilkington et
al. demonstrated that favipiravir had no serious side effects [56].
In other study the drug was reported to be safe and well-tolerated
in short-term use [57]. Chen et al. [35] reported that adverse
events are mild and manageable and the most frequently
observed adverse event was raised serum uric acid (16/116, OR:
5.52, P=0.0014). According to Ruzhentsova et al. and Zhao et al.,
the most common adverse events were asymptomatic hyperuricemia,
transient elevation of ALT and AST, and gastrointestinal
disorders (diarrhea, nausea, and abdominal pain). Balykova et
al. [32] also confirmed these results. Favipiravir treatment in 5
patients (13%) led to mild to moderate side events related to the elevations in hepatic enzymes, total bilirubin, uric acid and gastrointestinal
disorders [58]. Despite its anti-inflamatory activity
some ADEs suspected to be caused by favipiravir were reported.
They included increased hepatic enzymes, nausea and vomiting,
tachycardia, and diarrhea. Severe ADEs included blood and lymphatic
disorders, cardiac disorders, hepatobiliary disorders, injury
poisoning, and procedural complications. Serious ADEs were
more common among male subjects aged 64 and above (48%
vs 26%, respectively) [59]. Additionally, cutaneous adverse reactions
were reported in patients infected with COVID-19 [60,61].
Limitations of the Study
There are some limitations in the present meta-analaysis. First of
all, the sample size is low in each study. Second, the dosage and
duration of intervention with favipiravir are different. Third, viral
clearance is measured by RT-PCR, not quantitative. This approach
to determine viral clearance is considered to be quite unreliable
and has relatively low resolution. Fourth, the SOC arm included
only lopinavir/ritonavir+INFalfa1b, umifenovir (arbidol), baloxavir,
chloroquine and hydroxychloroquine/oseltamivir+enoxaparin.
Therefore, it is necessary to include more combinations
of drugs to evaluate the effectiveness and safety of favipiravir.
Additionally, there is no unification of clinical measures of the
effect of treatment. This makes comparative analysis somewhat
difficult. Moreover, different authors have different approaches
when evaluating adverse events, and therefore comparative
analysis should be interpreted carefully.
Conclusion
No unified approach could be used as standard for measuring
the effect of COVID-19 treatment. A wide variety of preparations
and approaches exists as reference in the comparative analysis of
favipiravir. The regimens of favipiravir administration are approximately
the same; difference is noted in the dosage and duration
of treatments. There is a significant difference in the clinical improvement
detected on Day 7 and day 14 in favour of favipiravir
over SOC or other treatments. A slightly higher chance for clinical
improvement exhists at Day 7 and Day 14. Viral clearance is expected
to be slightly higher by Day 7 of treatment with Favipiravir
and comparable to SOC thereafter at Days 7 and 14 is comparable
between treatments with neither being associated with significantly
better outcomes. The safety profiles of favipiravir and
SOC regarding SAE and SAE including death show no statistically
significant differences. It should be noted that different authors
have different approaches when evaluating adverse events and
therefore the comparative analysis should be interpreted carefully.
Author Contributions Statement
Y.Z. conceived and designed the analysis; contributed analysis
tools; conducted the analysis; drafted the paper and revised the
paper. F.C. conceived and designed the analysis; reviewed and
revised the paper; and managed the project. G.T. conceived and
designed the analysis; collected data; reviewed and revised the
paper. L.Y. reviewed and revised the paper; checked compliance.
All authors reviewed the manuscript.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
All data generated or analyzed during this study are included in
this published article [and its supplementary information files].
Competing Interests
VP, KU and EF are employees of Tchaikapharma High Quality
Medicines Inc. KK and TV have no relevant financial or non-financial
interests to disclose.
Funding
Tchaikapharma High Quality Medicines Inc. funded the current
meta-analysis.
Authors' Contribution
Conceptualization: [Toni Vekov, Krassimir Kalinov]; Methodology:
[Toni Vekov, Krassimir Kalinov]; Formal analysis and investigation;
[Velichka Pavlova, Katya Uzunova, Elena Filipova]; Writing-original
draft preparation: [Katya Uzunova, Velichka Pavlova]; Writing-
review and editing: [Elena Filipova]; Funding acquisition:
[Toni Vekov]; Supervision: [Toni Vekov, Krassimir Kalinov].
Acknowledgement
The authors wish to thank Ivona Danova for her support in language
editing.
References
- WHO (2020) Naming the coronavirus disease (covid-19) and the virus that causes it. World Health Organization (WHO).
- Corona Virus Research Centre. Johns hopkins Coronavirus resource center.
- Kumar P, Sah AK, Tripathi G (2021) Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in covid-19. Mol Cell Biochem 476: 553–574.
[Crossref] [Google Scholar]
- Poutanen SM (2012) Principles and practice of pediatric infectious diseases.
- Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G (2020) Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 4(4):66-75.
[Crossref] [Google Scholar]
- Cai Q, Yang M, Liu D (2020) Experimental treatment with favipiravir for covid-19: An open-label control study. Engg (Beijing, China) 6(10): 1192–1198.
[Crossref] [Google Scholar]
- Li G, De Clercq E (2020) Therapeutic options for the 2019 novel Coronavirus (2019-nCoV). Nat Rev Drug Discov 19(3): 149–150.
[Crossref] [Google Scholar]
- Al-Osail AM, Al-Wazzah MJ (2017) The history and epidemiology of Middle East respiratory syndrome Corona virus. Multidiscip Respir Med 12.
[Crossref] [Google Scholar]
- Huang Y (2004) The SARS epidemic and its aftermath in China: A political perspective. Institute of Medicine (US) Forum on Microbial Threats.
- Knobler S, Mahmoud A, Lemon S, Mack A, Sivitz L, et al. (2004) Learning from SARS: Preparing for the next disease outbreak: Workshop Summary. Washington (DC): National Academies Press (US).
- Ki M (2015) 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health 37: e201503337.
[Crossref] [Google Scholar]
- Saleki K, Yaribash S, Banazadeh M, Hajihosseinlou E, Gouravani A, et al. (2021) Interferon therapy in patients with SARS, MERS, and covid-19: A systematic review and meta-analysis of clinical studies. Eur J Pharmacol 906: 174248.
[Crossref] [Google Scholar]
- Kahn JS, McIntosh K (2005) History and recent advances in Coronavirus discovery. J Pediatr Infect Dis 24 (11 Suppl): S223–S226.
[Crossref] [Google Scholar]
- Wu YC, Chen CS, Chan YJ (2020) The outbreak of covid-19: An overview. JCMA 83(3): 217–220.
[Crossref] [Google Scholar]
- Larsen JR, Martin MR, Martin JD (2020) Modeling the onset of symptoms of covid-19. Front Pub Heal 8: 473.
[Crossref] [Google Scholar]
- Baj J, Karakuła-Juchnowicz H, Teresiński G (2020) Covid-19: Specific and non-specific clinical manifestations and symptoms: The current state of knowledge. J Clin Med 9(6): 1753.
[Crossref] [Google Scholar]
- Morris SB, Schwartz NG, Patel P (2020) Case series of multisystem inflammatory syndrome in adults associated with sars-cov-2 infection-United Kingdom and United States, march-august 2020. MMWR 69(40): 1450–1456.
[Crossref] [Google Scholar]
- Abdelmaksoud A, Kroumpouzos G, Jafferany M (2020) Covid-19 in the pediatric population. Dermatol Ther 33(4): e13339.
[Crossref] [Google Scholar]
- Han F, Liu Y, Mo M (2021) Current treatment strategies for Covid‑19 (Review). Mol Med Rep 24(6): 858.
[Crossref] [Google Scholar]
- Saul S, Einav S (2020) Old drugs for a new virus: Repurposed approaches for combating covid-19. ACS Infect Dis 6(9): 2304–2318.
[Crossref] [Google Scholar]
- Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019 (covid-19). Drug Disc Therapeu 14(1): 58–60.
[Crossref] [Google Scholar]
- Furuta Y, Takahashi K, Shiraki K (2009) T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antivir Res 82(3): 95–102.
[Crossref] [Google Scholar]
- Furuta Y, Gowen BB, Takahashi K (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res 100(2): 446–454.
[Crossref] [Google Scholar]
- Sleeman K, Mishin VP, Deyde VM (2010) In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A (H1N1) viruses. Antimicrob Agents Chemother 54(6): 2517–2524.
[Crossref] [Google Scholar]
- Agrawal U, Raju R, Udwadia ZF (2020) Favipiravir: A new and emerging antiviral option in COVID-19. Med J Armed Forces India 76(4): 370–376.
[Crossref] [Google Scholar]
- Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan academy. Phy bio sci 93(7): 449–463.
[Crossref] [Google Scholar]
- Coomes EA, Haghbayan H (2020) Favipiravir, an antiviral for covid-19? J Antimicrob Chemother 75(7): 2013–2014.
[Crossref] [Google Scholar]
- Shiraki K, Daikoku T (2020) Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther 209: 107512.
[Crossref] [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y (2002) In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother 46(4): 977–981.
[Crossref] [Google Scholar]
- Lee JS, Adhikari N, Kwon HY (2019) Anti-Ebola therapy for patients with Ebola virus disease: A systematic review. BMC Infect Dis 19(1): 376.
[Crossref] [Google Scholar]
- Oestereich L, Lüdtke A, Wurr S (2014) Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir Res 105: 17–21.
[Crossref] [Google Scholar]
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Fontela CM, et al. (2014) Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir Res105: 17-21.
[Crossref] [Google Scholar]
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30(3): 269-271.
[Crossref] [Google Scholar]
- Balykova L, Pavelkina V, Shmyreva N, Pyataev NA, Selezneva NM, et al. (2020) Efficacy and safety of some etiotropic therapeutic schemes for treating patients with novel coronavirus infection (covid-19). Pharm Pharmacol 8(3): 150-159.
[Crossref] [Google Scholar]
- Cai Q, Yang M, Liu D, Chen J, Shu D, et al (2020) Experimental treatment with favipiravir for covid-19: an open-label control study. Engineering (Beijing, China) 6(10): 1192–1198.
[Crossref] [Google Scholar]
- Udwadia ZF, Singh P, Barkate H, Patilb S, Rangwala S, et al. (2021) Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. IJID103: 62–71.
[Crossref] [Google Scholar]
- Chen C, Zhang Y, Huang J, Yin P, Cheng Z, et al. (2020) Favipiravir versus arbidol for covid-19: A randomized clinical trial. MedRxiv
[Crossref] [Google Scholar]
- Dabbous HM, El-Sayed MH, Assal Gel, Elghazaly H, Ebeid FFS, et al. (2020) A randomized controlled study of favipiravir vs hydroxychloroquine in Covid-19 management: What have we learned so far? Research Square.
[Crossref] [Google Scholar]
- Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, et al. (2021) AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (covid-19): Interim results of a phase ii/iii multicenter randomized clinical trial. CID 73(3): 531–534.
[Crossref] [Google Scholar]
- Lou Y, Liu L, Yao H, Hu X, Su J, et al. (2021) Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in covid-19 patients: An exploratory randomized, controlled trial. Eur J Pharm Sci157:105631.
[Crossref] [Google Scholar]
- Ruzhentsova T, Chukhliaev P, Khavkina D, Garbuzov A, Oseshnyuk R, et al. (2020) Phase 3 trial of coronavir (Favipiravir) in patients with mild to moderate covid-19. SSRN.
[Crossref] [Google Scholar]
- Zhao H, Zhang C, Zhu Q, Chen X, Chen G, et al. (2021) Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, open-label, randomized trial. Int Immunopharmacol 97: 107702.
[Crossref] [Google Scholar]
- Dabbous HM, Abd-Elsalam S, El-Sayed MH, Sherief AF, Ebeid FFS, et al. (2021) Efficacy of favipiravir in Covid-19 treatment: A multi-center randomized study. Arch Virol 166(3): 949–954.
[Crossref] [Google Scholar]
- Hassanipour S, Arab‑Zozani M, Amani B, Heidarzad F, Fathalipour M, et al. (2021) The efficacy and safety of Favipiravir in treatment of Covid‑19: A systematic review and meta‑analysis of clinical trials. Sci Rep 11(1): 11022.
[Crossref] [Google Scholar]
- Manabe T, Kambayashi D, Akatsu H, Kudo K (2021) Favipiravir for the treatment of patients with covid-19: A systematic review and meta-analysis. BMC Infect Dis 21: 489.
[Crossref] [Google Scholar]
- Özlüşen B, Koozan Ş, Akcan RE, Kalender M, Yaprak D, et al. (2021) Effectiveness of favipiravir in covid‑19: A live systematic review. Eur J Clin Microbiol Infect Dis 11: 11022.
[Crossref] [Google Scholar]
- Qomara WF, Primanissa DN, Amalia SH, Purwadi FV, Zakiyah N, et al. (2021) Effectiveness of remdesivir, lopinavir/ritonavir, and favipiravir for covid-19 treatment: A systematic review. Int J Gen Med 14: 8557-8571.
[Crossref] [Google Scholar]
- Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, et al. (2020) Favipiravir versus other antiviral or standard of care for COVID‑19 treatment: A rapid systematic review and meta‑analysis. Virol J 17(1): 141.
[Crossref] [Google Scholar]
- Prakash A, Singh H, Kaur H, Semwal A, Sarma P, et al. (2020) Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (covid-19) patients. Indian J Pharmacol 52(5): 414–421.
[Crossref] [Google Scholar]
- COVID-19 Treatment Guidelines Panel (2022) Coronavirus disease 2019 (covid-19) treatment guidelines. National institutes of health.
- World Health Organization (2022) The international guidelines for the management of Coronavirus disease 2019 developed by the World Health Organization (WHO).
- Yamakawa K, Yamamoto R, Terayama T, Hashimoto H, Ishihara T, et al. (2021) Japanese rapid/living recommendations on drug management for Covid-19: Updated guidelines (September 2021). Acute Med Surg 8: e706.
[Crossref] [Google Scholar]
- Australian guidelines for the clinical care of people with covid-19.
- Philippine Covid-19 living clinical practice guidelines.
- Interim clinical guidance for adults with suspected or confirmed Covid-19 in Belgium.
- UAE, ministry of health and prevention. National guidelines for clinical management and treatment of covid-19.
- Pilkington V, Pepperrell T, Hill A (2020) A review of the safety of favipiravir-a potential treatment in the Covid-19 pandemic? J Virus Erad 6(2): 45–51.
[Crossref] [Google Scholar]
- Malvy D, Taburet AM, de Lamballerie X, Mentre F, Extramiana F, et al. (2020) The safety profile of favipiravir should not be the first argument to suspend its evaluation in viral hemorrhagic fevers. PLOS Negl Trop Dis 14(6): e0008259.
[Crossref] [Google Scholar]
- Erdem HA, Korkma PE, Çağlayan D, Taşbakan MI, Yamazhan T, et al. (2021) Treatment of SARS-CoV-2 pneumonia with favipiravir: Early results from the Ege university cohort, Turkey. Turk J Med Sci. 51(3): 912–920.
[Crossref] [Google Scholar]
- Kaur RJ, Charan J, Dutta S, Sharma P, Bhardwaj P, et al. (2020) Favipiravir use in Covid-19: Analysis of suspected adverse drug events reported in the who database. Infect Drug Resist 13: 4427–4438.
[Crossref] [Google Scholar]
- Punyaratabandhu P, Vanitchpongphan S (2021) Favipiravir-induced cutaneous adverse reactions in patients infected with covid-19. Clin Exp Dermatol 47(3):573-577.
[Crossref] [Google Scholar]
Citation: Pavlova V, Uzunova K, Filipova E, Kalinov K, Vekov T (2022) Repurposing of Favipiravir for the Treatment COVID-19: A
meta-analysis. J Infect Dis treat. 9:05.
Copyright: ©2023 Pavlova V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source
are credited.






