- (2014) Volume 15, Issue 6
Pablo E Serrano1, Stefano Serra2, Hassan Al-Ali3, Steven Gallinger3, Paul D Greig3, Ian D McGilvray3, Carol-Anne Moulton3, Alice C Wei3 and Sean P Cleary3
1Department of Surgery, McMaster University, Hamilton, 2Department of Pathology, 3Surgery, University Health Network, University of Toronto, Toronto, Canada
Received June 7th, 2014 – Accepted September 25th, 2014
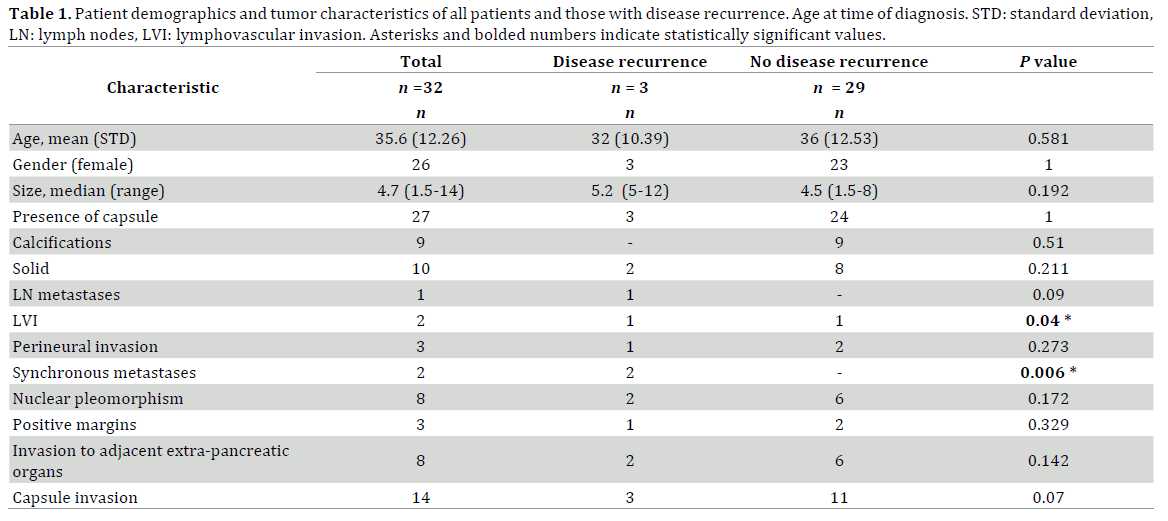
Context Solid pseudopapillary tumors (SPT) are rare, generally low grade pancreatic neoplasms that occasionally display malignant behavior. Objective To analyze the clinical and pathological features associated with increased risk of recurrence of SPT. Methods Cohort study of patients with SPT who underwent resection of the primary tumor and in selected cases resection of metastatic disease from 1999-2013 at a single tertiary care Hepatopancreatobiliary center. Risk factors for recurrence were statistically analyzed. Results There were 32 patients. The mean age was 35.65 years (standard deviation: 12.26), 26/32, 81.25% were female. Median size of resected tumors was 4.7cm (1.1-14.5). Most were solid and cystic (22/32, 68.75%), encapsulated (27/32, 84.4%) and located in the pancreatic body or tail (22/32, 68.75%). All displayed strong β-catenin, cyclin D1, CD56, and progesterone receptor staining with loss of E-cadherin. Most stained positive for vimentin (15/16, 93.75%) and CD10 (17/18, 94.4%). Median follow-up was 43 months (range: 3-207); 3/32, 9.38% recurred (all after 5-years from curative resection) and 1 died by the end of the study period, 11 years after diagnosis. Patients who developed recurrences (n=3) more commonly had synchronous metastases at presentation (P =0.006), lymphovascular invasion (P=0.04) and invasion of tumor capsule (P=0.08) compared to those who did not have disease recurrence. Conclusions Lymphovascular invasion, synchronous metastases and local invasion of tumor capsule are associated with aggressive behavior. Since recurrences may occur > 5 years from resection, this high-risk group should undergo extended follow-up. Progression and recurrence is slow, therefore, resection of liver metastases can offer long-term survival.
Pancreas, Exocrine; Pancreatectomy; Pancreatic Neoplasms
Solid pseudopapillary tumors (SPT) of the pancreas, initially described by Frantz in 1959 [1], later included in the World Health Organization classification in 1996 by Kloppel et al, [2-4], are rare pancreatic neoplasms with uncertain potential for malignancy. These tumors are commonly found in women [5, 6], and have been traditionally recognized as having low malignant potential. Pooled data shows that overall 5-year survival is excellent (>95%), even in those patients with liver metastases (15- 20%) [7].
Despite the well-defined histopathological and immunohistochemical features of SPT, their pathogenesis and cellular derivation remains unclear [8]. The phenotypic characteristics are different from any other normal epithelial cell lines of the pancreas. In contrast to pancreatic adenocarcinomas, SPT do not show p53 or K-ras genetic alterations [9, 10] but rather their tumorigenesis seems to be associated with abnormalities in the Wnt signal transduction pathway with p120 catenin mutations, resulting in complete loss of membrane expression of E-cadherin and nuclear localization of β-catenin [11-14].
Factors that have been associated with increased risk of recurrence and metastases include the presence of perineural and lymphovascular invasion, high mitotic rate and cellular pleomorphism (originally described by Koppel et al. as solid pseudopapillary carcinoma) [2, 15]. Previously reported case series and systematic reviews of retrospective studies have evaluated the risk of recurrence in patients with SPT; however the retrospective nature of these studies limits the validity of the findings. Previously reported case-series and systematic reviews previously published in the literature have tried to evaluate the risk of recurrence in patients with SPT; however results are limited by the retrospective review of the studies and the small number of patients included in them [16-19].
The main objective of this study was to identify risk factors for disease recurrence in patients with SPT of the pancreas in order to help determine follow-up surveillance recommendations for this patient population. This study will provide one of the largest western series of patients with SPT treated at a single hepatopancreatobiliary tertiary care center.
Selection of Participants and Data Collection
This study describes the clinic-pathological and surgical characteristics of patients with a diagnosis of SPT of the pancreas at a single tertiary care Hepatopancreatobiliary center from 1999 to 2013. Records were obtained from the Performance Measurement Department database and the Hepatopancreatobiliary and Pathology database after obtaining approval by the institutional research ethics board. All patients undergoing pancreatectomy during this period were reviewed and those with a pathological diagnosis of SPT of the pancreas were included in the analysis.
Data, including demographic, radiologic, perioperative and pathological variables were retrospectively collected from electronic medical records. All patients were followed at the oncology clinics every 6 months to 1 year with history, physical examination and imaging, either with computed tomography (CT), magnetic resonance imaging (MRI) or ultrasonography. The date and the cause of death were obtained from provincial vital statistic records.
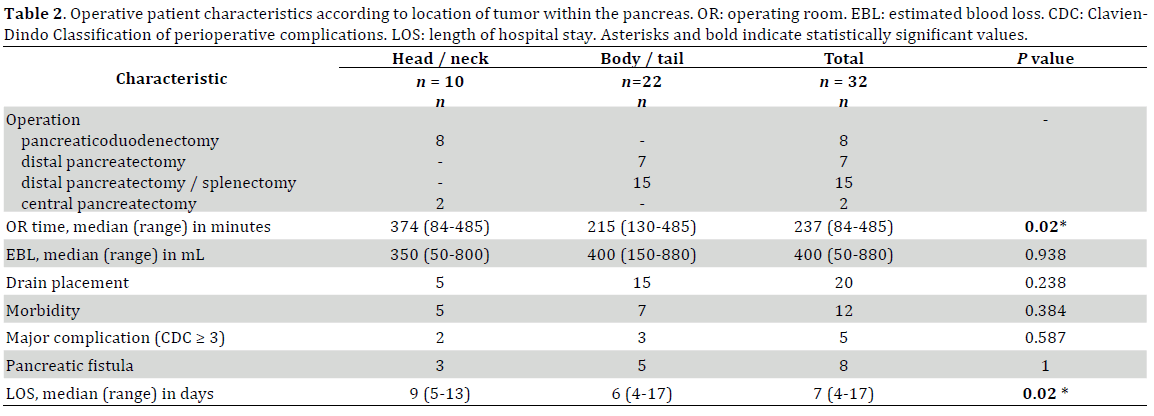
All pancreaticoduodenectomies and central pancreatectomies were performed via open approach. For distal pancreatectomies, the decision to perform an open vs. laparoscopic technique was at the discretion of the operating surgeon. After the year 2005, a minimally invasive approach was chosen for distal pancreatectomies when the suspected preoperative diagnosis was SPT, neuroendocrine tumor or cystic neoplasm of the pancreas. In cases of suspected adenocarcinoma, an open approach was preferred. Intraoperative frozen sections were not routinely performed in all cases. Intraoperative peritoneal drain placement was variable and depended on surgeons’ preference.
Post-operative complications were graded according to the Clavien-Dindo scale adapted for pancreas surgery [20]. Any complication of grade III or higher was classified as a major complication. The definition for pancreatic leak / fistula was adopted from the International Study Group on Pancreatic Fistula (ISGPF) [21]; specifically: any output of amylase rich fluid (amylase level 3 times the upper limit of normal) from a surgical drain (or subsequently placed percutaneous drain) on or after post operative day 3 was taken as evidence for a leak. Grade A pancreatic fistula has no clinical impact. Grade B fistula requires change in the management and the patient’s clinical course. Grade C is a significant fistula that results in major alteration of clinical course [21].
Pathologic analysis
The diagnosis of SPT was based on histopathologic and immunohistochemical evaluation. Details of institutional pathologic assessment for SPT have been published previously [12, 22]. In summary, most slides were stained with CD10, progesterone receptor, α1- antitrypsin, synaptophysin, chromogranin and vimentin as part of the routine workup for these tumors. Additional stains, performed on a per-case basis included: EMA, p53, cytokeratin (AE1/AE3 and Cam 5.2), neuron specific-enolase, low molecular weight keratin (LMWK), somatostatin, pancreatic polypeptide, insulin, glucagon, estrogen receptors, CK7, CK19, CK20, TTF-1, CD-57, CD56, β-catenin, e-cadherin loss of staining, cyclin D1 and α-fetoprotein.
Standard evaluation of pathological specimens included: size and location of the tumor within the pancreas, presence of capsule and calcifications. Pathologic staging was reported according to the seventh edition of the TNM staging system supported by the International Union Against Cancer and American Joint Committee on Cancer (AJCC) for solid exocrine pancreatic tumors and correlated for the purposes of this study with the WHO classification of SPT by Kloppel, et al. [2, 23, 24]. Margin status, presence of lymphovascular and perineural invasion was also reported. Tumor size was defined as the maximum crosssectional diameter at the time of pathological evaluation.
Data analysis
Categorical variables were expressed as counts and proportions, while continuous variables were expressed as medians and range. Age has been presented as means and standard deviation. Differences between categorical variables were calculated using chi-square or Fisher’s exact test when appropriate. Non-parametric variables were compared using Kruskal-Wallis rank sum test. Disease-free survival (DFS) was defined as the time from complete surgical resection to local or distant relapse and was calculated using the Kaplan-Meier method. Date of recurrence was defined as the date of documentation by diagnostic imaging techniques of recurrent disease. Followup was defined as time from diagnosis to last clinical visit at the end of the study period. A P value of less than 0.05 was considered statistically significant. Calculations were performed using R, version 2.15.2.
Participant characteristics
Of 1254 pancreatic resections performed during the study period, there were 32 (2.5%) with a diagnosis of SPT and were included in this analysis. Most were females (26/32, 81.25%). Mean age at diagnosis was 35.6 years (standard deviation: 12.26) (Table 1). A majority of patients had symptoms at presentation (20/32, 62.5%); most of these were non-specific abdominal (17/32, 53.13%) or back pain (3/32, 9.38%). None of these patients presented with jaundice, diabetes, weight loss or pancreatitis.
Preoperative diagnosis of SPT was made in 18/32, 56.25% of patients while the remainder had undergone resection for other presumed pathology including neuroendocrine tumors (4/32, 12.5%), mucinous cystic neoplasms (8/32, 25%) and pancreatic cancer (2/32, 6.25%). CT as the sole imaging test was used in 18/32, 56.25%, MRI in 5/32, 15.63% and both methods in 9/32, 28.12%. When preoperative diagnosis of SPT was not clear, other diagnostic modalities were used, including: octreotide scan, contrast enhanced ultrasound and preoperative biopsy either percutaneous or via endoscopic ultrasound.
Operative interventions and postoperative outcomes
There were 8 pancreaticoduodenectomies, 2 central pancreatectomies, 7 spleen-preserving distal pancreatectomies, and 15 distal pancreatectomies with splenectomy. There were 11/21, 52.4% completely laparoscopic distal pancreatectomies. (Table 2.) There were 2 further laparoscopic distal pancreatectomies converted to open, one due to bleeding and another one because of invasion of stomach and colon. Enbloc resection of adjacent extra-pancreatic organs was performed when direct invasion of the tumor was found; this included 3 partial colon resections, 1 partial stomach resection, 2 partial resection of retroperitoneal skeletal muscle, 2 partial resection of Gerota’s fascia and kidney and 1 resection of spleen due to direct involvement of the hilum. Two patients with adjacent extra-pancreatic organ invasion also had liver metastases (Tables 1 and 3).
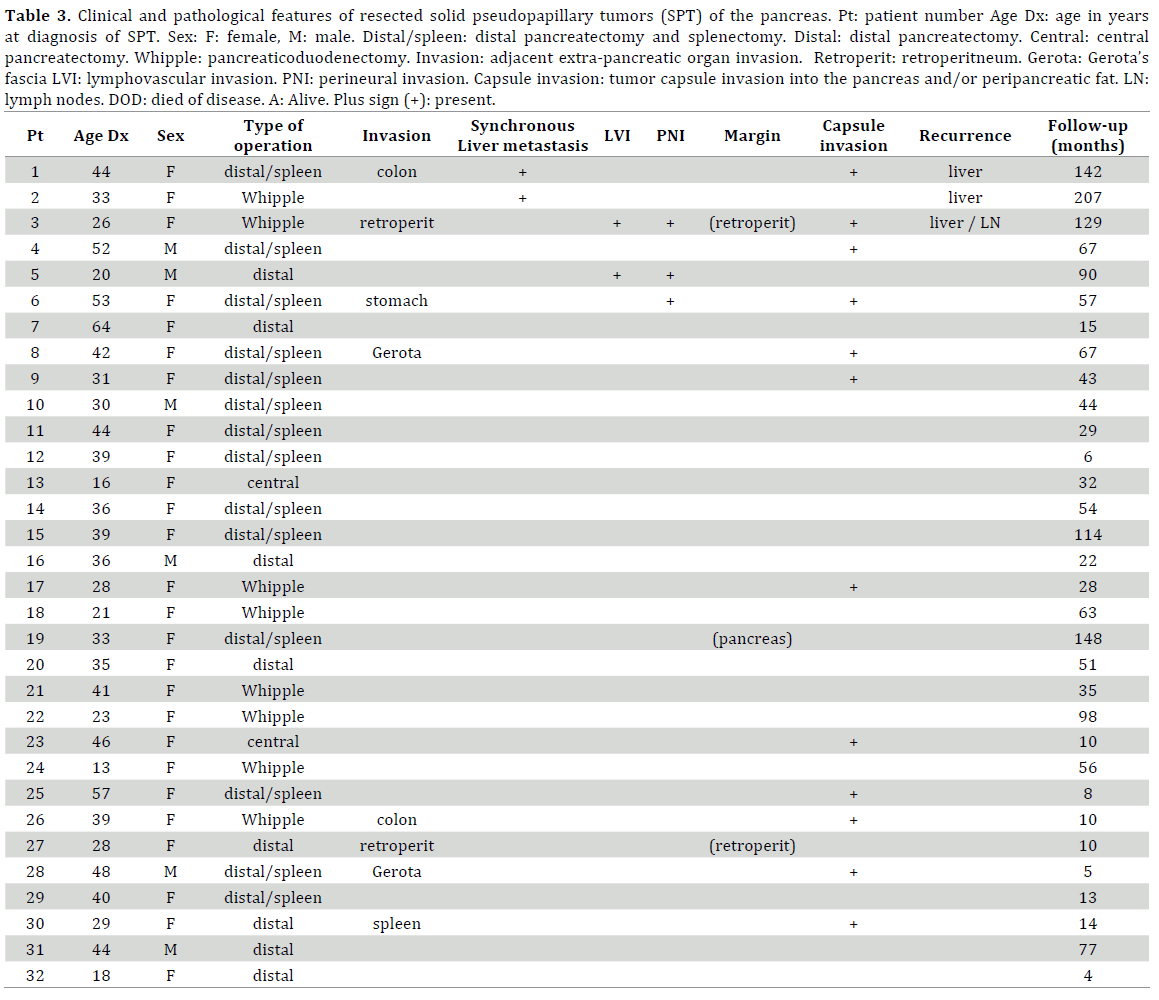
Recurrent liver metastases developed in a total of 3 patients; two patients had synchronous metastases while the third patient developed metastatic disease 6 years after primary tumor resection (margins and lymph nodes were positive at time of pancreatic resection) (Table 1).
Patient 1 was a 44 year-old female who underwent a right trisectionectomy for metastatic SPT 5 months following open distal pancreatectomy and partial colon resection for colon invasion [25]. On further follow-up, 7 years later there was recurrence in segment IV-a that was unsuccessfully treated twice with RFA, eventually undergoing segment IV-a wedge resection 9 years after initial operation. Unfortunately there was evidence of retroperitoneal and intra-hepatic recurrence 1½ years later, at which time an excision of retroperitoneal tumor and a wedge resection of segment III was performed. At the end of the study period, there was no evidence of disease recurrence. Patient 2 (33 year-old, female) underwent a simultaneous segment II resection at the time of pancreaticoduodenectomy with subsequent right hepatectomy after 2months and a further wedge resection of segment III and IV 5 years later. This patient was disease free 5 years from last liver resection. The third patient (Patient 3, 26 year-old, female) was lost to follow-up after pancreaticoduodenectomy and found to have disseminated metastatic disease (retroperitoneal lymph nodes and extensive liver metastases) 6 years later and was not offered resection. Her original pancreatic tumor had lymphovascular and perineural invasion, positive lymph nodes (2/16, 12.5%) and positive resection margins in the retroperitoneal skeletal muscle. She died 5 years later after undergoing different courses of chemotherapy including gemcitabine and erlotinib.
Overall complication rate was 38% (12/32) and the major complication rate was 16% (5/32) (Table 2). The most common complication was pancreatic fistula, seen in 8/32, 25% patients; most of them were categorized as Grade A (5/8, 62.5%) without any Grade C. The remaining postoperative complications were bleeding with need for reoperative intervention (1/32, 3.13%), intra-abdominal abscess resolving with antibiotics and percutaneous drainage (2/32, 6.25%), pulmonary embolism (1/32, 3.13%), urinary tract infections (2/32, 6%) and wound infections (2/32, 6%).
Median follow-up was 43 months (range, 3-207). Last follow-up date for the patient who was followed up the longest was March 2013. Median follow-up from diagnosis to surgical resection was 3 months (range 1-83 months) and the mean follow up was 11.5 months (standard deviation 20.7). At the conclusion of the study, only one patient had died (Patient 3), the remaining were alive without evidence of disease recurrence. Median DFS was 88 months (95% confidence interval: 64 – not reached).
Pathology assessment and immunohistochemistry
There were three patients with positive resection margins, two of them the posterior retroperitoneal margin and the other one, the pancreatic resection margin. One of these patients with positive margins (Patient 3) developed disseminated metastatic disease 6 years after pancreaticoduodenectomy. Most tumors were large but totally or partially encapsulated within the pancreas. Invasion through the capsule into pancreatic or peripancreatic fat was seen in 14/32, 43.75% patients. Final AJCC staging showed that most tumors (21/32, 65.63%) were Stage 1 and most belonged to a T2 stage, >2 cm and limited to the pancreas (20/32, 62.5%). Lymphovascular invasion was present in two patients; one of them (Patient 3) also with lymph node metastases, eventually developed disseminated metastatic disease. The other patient has had no evidence of recurrence at the conclusion of the study (follow up: 90 months). For all patients, the median number of lymph nodes collected was 7 (range, 2-59). Perineural invasion was present in three patients, including Patient 3; the other two patients are still alive 129 and 90 months later without evidence of recurrence or metastases. There were no major histopathological differences between patients with and without invasion of tumor capsule or adjacent extra-pancreatic organs (data not shown). Patient who developed disease recurrence however, more commonly presented with lymphovascular invasion (P=0.04), synchronous metastases (P=0.006) and invasion of tumor capsule into the peri-pancreatic fat (P=0.07).
Histologically, SPT are characterized by a distinctive appearance of solid and cystic areas with the formation of pseudopapillary structures. The solid areas consist of uniform polygonal cells with eosinophilic cytoplasm, round to oval nuclei with finely stippled chromatin and frequent grooves admixed with thin delicate capillaries without gland formation. These cells commonly have intracytoplasmic vacuoles or eosinophilic hyaline globules. The cystic areas are had of prominent degenerative changes with pseudopapillae characterized by loosely cohesive cells surrounding delicate capillary-sized blood vessels. (Figure 1) [26] Areas with foamy macrophages, hemorrhage and cholesterol crystals were seen. All tumors were diffusely positive for neuron specific enolase, progesterone receptor, α-1 antitrypsin, α-1 antichymotrypsin, CD10, CD56, CD57 and cyclin D1. Immunoreactivity for β-catenin was found in the cytoplasm and the nuclei of almost all tumor cells. There was loss of e-cadherin staining in all analyzed slides. All but one patient (Patient 3) were positive for vimentin. Epithelial markers were less commonly positive, cytokeratin Cam 5.2 (2/7, 28.5%), cytokeratin AE1-AE2 (2/8) and low molecular weight keratin (LMWK) (3/7, 42.85%). Endocrine markers varied, with synaptophysin being positive in 12/20, 60% and chromogranin was focally positive (dot-like) in two patients (2/20, 10%).
Figure 1. Histopathology and immunohistochemistry of SPT. a. Hematoxylin and eosin (H&E) stain of solid tumor areas demonstrating uniform polygonal cells with eosinophilic neoplasm, round nuclei with finely stippled chromatin and frequent groves admixed within capillaries. b. H&E stain of cystic tumor areas demonstrating mixed cysts characterized by degenerative changes with formation of pseudopapillary structures. c. Immunoreactivity for β-catenin shows cytoplasmic and nuclei positivity in most tumor cells. d. Vimentin staining is positive in almost all tumor cells.
SPT are uncommon neoplasms, accounting for less than 1-2% of all pancreatic tumors and are mostly encountered in females in the body or tail of the pancreas. [27] This study analyzed one of the largest single institution series of resected SPT and showed the clinical and pathological characteristics of this patient population. Similar to previously reported studies [28], we showed that prognosis is excellent and the risk for recurrence is small. [29-32] Although patients usually present with vague abdominal complaints or symptoms, none presented with weight loss, jaundice or diabetes even if large or located in the head/neck of the pancreas, distinguishing them from truly malignant tumors [33, 34]. Very few patients presented as an incidental finding, in contrast to most pancreatic cystic neoplasms [35].
Even though SPT have typical radiological features, including a large encapsulated mass with solid and cystic components and intra-tumoral hemorrhage [30, 36, 37], more than 40% of were not appropriately diagnosed preoperatively, with most being mistaken for pancreatic cystic neoplasms. It is not possible to predict aggressive behavior on the basis of imaging findings. These results are somewhat different from the findings by Salvia, et al in which most SPT were diagnosed preoperatively with diagnostic imaging [30, 38]. Accuracy of imaging modalities (especially CT and MRI) has improved tremendously over the past two decades [39]. In our study, most SPT were mistaken for other cystic neoplasms of the pancreas.
Irrespective of their location, most tumors were large, encapsulated and contained within the pancreas; almost all of them had a final AJCC stage 1 (21/32, 65.6%) or 2, (8/32, 25%) with the vast majority being the former. SPT are usually well demarcated with a soft white-grey to yellow solid component and often a central cavity, containing friable necrotic material and areas of recent or remote hemorrhage. Less frequently they can show an infiltrative pattern of growth with invasion into the surrounding pancreatic tissue [27]. Margins were positive in three patients, two of them had invasion to retroperitoneal structures. Frank malignant behavior with metastases to other organs or lymph nodes was seen in two of these patients that had extra-pancreatic organ invasion. Lymphovascular invasion was also another feature that suggested malignancy while perineural invasion was not consistently associated with metastases or recurrence.
Most SPT can be readily diagnosed by routine histologic examination, although immunohistochemical studies are frequently performed to confirm diagnosis. Consistently, SPT are vimentin, CD10, CD56 and progesterone receptor positive and have nuclear β-catenin expression [11, 16, 22]. Interestingly, the only patient in our series that died from disseminated metastatic disease was vimentin-negative. None of these immunohistochemical characteristics were associated with malignant behavior. SPT neoplastic cells have bland cytological appearance and low mitotic activity, even in the presence of metastatic disease. This compares to the findings by Tang et al. where 5 of the 7 patients with SPT and liver metastases did not exhibit significant increased cellularity, nuclear pleomorphism or high (>30%) Ki-67 and mitotic rate (35-70/50 HPF).
Due to their malignant potential, surgical resection remains the standard treatment for SPT [40, 41]. The overall and major complication rate is not trivial, independent of the location and type of surgery performed. The rate of pancreatic fistula, the most common complication seen, was higher than most published series [42] of pancreatectomies (pancreaticoduodenectomy and distal pancreatectomy) including our own institutional experience over the same period (12%, data not shown). Even though large, SPT do not usually obstruct the pancreatic duct, therefore most cases have a soft gland texture and a small pancreatic duct size, classic risk factors for postoperative pancreatic fistula [43, 44]. Since the majority of SPT do not invade other organs, laparoscopic distal pancreatectomy is a safe and attractive option for resection. Most cases where preoperative diagnosis was not clear were thought to be pancreatic cystic neoplasms for which laparoscopy is the general approach at this institution.
Pancreatic sparing resections, including central pancreatectomy is also a reasonable option as these tumors tend to be encapsulated and well defined within the pancreas even in those where invasion beyond the capsule and into surrounding pancreatic parenchyma was found. [45] Positive margins were not associated with risk of recurrence after resection of SPT. Extended lymphadenectomy does not seem to be an important component of the management of these tumors as the risk of lymph nodes metastases is very low.
Given the relatively low incidence of these tumors, guidelines for clinical and radiological follow-up have not yet been clearly defined. Despite the relatively low number of patients of this cohort, there were three patients with disease recurrence; two of them had synchronous metastases at diagnosis. Recurrence occurred 5 to 7 years following complete surgical excision. Recurrence was distant (liver) in all three patients and local (retroperitoneum / pancreatic bed) in two of the three. Surgical resection of disease recurrence provided good long-term results for one of the patients (Patient 2), the other (Patient 1) has undergone three further surgical excisions of recurrent disease at 7, 2 and 1.5 years apart. Previous published reports have found similar late recurrence patterns [8, 28, 29]. This seems to indicate that > 5-year clinical follow-up is warranted after resection of SPT with high-risk features (lymphovascular invasion, synchronous metastases and perhaps invasion of tumor capsule). Since this is a slow growing tumor, resection of recurrences and metastases can offer good long-term survival. Even with metastatic disease to the liver, these patients have better survival rates than other types of pancreatic cancer.
Due to the low but real malignant potential, all SPT should be resected open or laparoscopically, with no oncological disadvantages found in the latter group for distal pancreatectomies. Lymphovascular invasion and synchronous metastases represent a high-risk group for recurrence. Since all recurrences occurred more than 5 years after curative resection, patients in this high-risk category should undergo > 5-year follow-up surveillance with routine imaging, especially considering that resection of recurrences (either distal or local) can offer long-term cure.
There are no conflicts of interest to disclose for this study. We attest that we have herein disclosed any and all financial or other relationships that could be construed as a conflict of interest and that all sources of financial support for this study have been disclosed and are indicated in the acknowledgements.
Authors declare to have no conflict of interest.