Research Article - (2024) Volume 32, Issue 4
Received: 24-Jul-2020, Manuscript No. IPQPC-24-5346; Editor assigned: 29-Jul-2020, Pre QC No. IPQPC-24-5346 (PQ); Reviewed: 12-Aug-2020, QC No. IPQPC-24-5346; Revised: 01-Jul-2024, Manuscript No. IPQPC-24-5346 (R); Published: 29-Jul-2024, DOI: 10.36648/1479-1064.32.3.15
Objective: To identify evidence in the scientific literature about pharmacological therapy for COVID-19 in pediatrics.
Methods: This is an integrative literature review, conducted using the spider tool and performed in June 2020, at PubMed using the descriptors “coronavirus infections, child and drug therapy”. After filtering from the inclusion and exclusion criteria, five articles summarized in synoptic tables were used and analyzed using an instrument.
Results: The selected articles pointed to the use of antiviral drugs (Lopinavir-Ritonavir (LPV/r), antibiotics (Vancomycin and Amikacin), antimalarial (hydroxychloroquine), immunomodulatory (interferon α-1b) and herbal medicine (lianhuaqingwen) in the pharmacological management of children with COVID-19.
Conclusion: Because it is a new strain of coronavirus, it is necessary to conduct clinical studies that guide evidence-based practice.
Evidence-based medicine; Child health; Coronavirus infections; Pharmacological treatment
Severe Acute Respiratory Syndrome by Coronavirus (SARSCoV- 2) has been the focus of global attention since several cases of pneumonia patients were identified in Wuhan-Hubei, China, in late December 2019.
Characterized as highly contagious, COVID-19 can be classified as mild, common, severe and critical. The first two types have a good prognosis, however the latter can result in systematic organic dysfunction, which characterizes the urgency to find appropriate drugs.
The clinical manifestations of the new coronavirus include fever, non-productive cough, dyspnoea, tachypnea, myalgia, fatigue and diarrhea and although they can affect all age groups, as pointed out by different studies, the manifestations are milder in children than in adults. It is noteworthy, however, that children's symptoms are different from adults in the context of respiratory infections, which brings in tow the need to pay more attention to children with COVID-19. Although coronavirus pneumonia is a serious disease with a high lethality rate, to date, there are no clinically effective pharmacological treatments proven against COVID-19, although several studies are underway. Care for patients with this problem is restricted to symptomatic treatment, being pointed out as support measures for oxygen administration, intravenous therapy, hydro electrolytic correction and acidbase disorders.
Given the epidemiological importance of the new coronavirus, it is urgent to find appropriate drugs for this problem. Therefore, research on pharmacological management for COVID-19 is important, given that these studies have the potential to contribute to patient care until a specific drug for the new coronavirus is developed, favoring the practice evidence-based clinic, with direct effects on the user's health and quality of life.
Given the above, this study aimed to identify what the scientific evidence about pharmacological therapy for COVID-19 in pediatrics points to.
Integrative literature review conducted in June 2020 on scientific evidence about pharmacological therapy for COVID-19 in pediatrics. It is an important method because it allows for a broad and systematic analysis of the literature on a given topic, favoring the investigation of what has already been published, the existing gaps and the dissemination of data produced by other authors.
To guide the elaboration of the research question and conduct the searches, the SPIDER tool was used, which consists of five elements: Sample (sample), phenonemon of interest (phenomenon of interest), design (study design), evaluation (evaluation) and research type. Therefore, the following guiding question was formulated: What is the scientific evidence (E) about the drugs used to treat COVID-19 (PI) in children (S) considering the phases of the integrative review, the elaboration of the research question (PI) was succeeded by the following actions: Search or sampling in the literature, anchored by inclusion and exclusion criteria (S/D/R); data collection (S); critical analysis of the included studies (E); discussion of results (E); and presentation of the review.
Data collection was carried out in three stages. The first consisted of surveying the studies on the PubMed portal using the descriptors “coronavirus infections, child and drug therapy”, consulted in the Medical Subject Headings (MeSH), of the national library. The descriptors were crossed by the use of the boolean operator and: Coronavirus infections and child and drug therapy. There was no restriction in the selection, considering the option “all fields” in the searches [1].
108 papers were found, filtered by the following inclusion criteria: Articles published in full, available electronically, in English. It is noteworthy that due to the fact that the new coronavirus is an incipient theme, only studies published in the year 2020 were selected. Articles whose results did not favor the drug treatment of children diagnosed with COVID-19 were excluded. From the inclusion criteria described, 71 articles were discarded (10 because they did not have the full text; 60 because they were not related to SARS-CoV-2, but to other types of coronavirus and 1 because it was not in English) and pre-selected 37.
The second stage of data collection was guided by a protocol prepared by the authors. Thus, the 37 pre-selected articles underwent a careful reading of the title and summary and studies that had children among the subjects and that dealt with drugs directed to infection by the new coronavirus in this public were selected. This new selection resulted in 20 articles.
In the third stage of the collection, which was also based on the aforementioned protocol, the 20 articles initially selected were subjected to a new analysis, in which the entire study was carefully read. After that, 15 articles were excluded because they did not specifically address drugs in the context of the new coronavirus and 5 works were selected to compose the corpus of this review. The process of survey, analysis and selection of studies is shown in the flowchart shown in Figure 1.
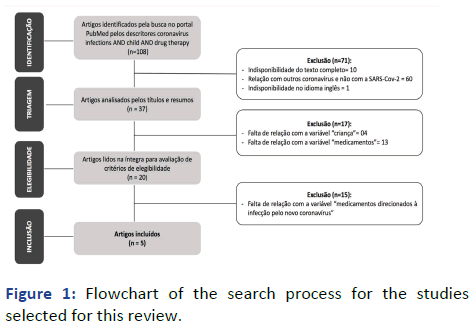
Figure 1: Flowchart of the search process for the studies selected for this review.
The selected articles were further evaluated and classified according to the level of evidence considering the system proposed by the oxford center for evidence-based medicine, in which the evidence is classified as 1a, 1b, 1c, 2a, 2b, 2c , 3a, 3b, 4 and 5.
As it did not involve human beings, it was not necessary to appraise and approve the study by a research ethics committee. It should be noted, however, that obeying law number 9.610, of February 19, 1998, which regulates copyright, the ethical principles regarding the preservation of authorship were respected. Thus, the consulted authors were duly cited.
The 5 selected articles were read in their entirety and after this, an instrument was filled with a summary of the studies regarding the following information: Identification (ID), authorship, title, journal, objective, type of study and sample, which found in Table 1. In Table 2 the level of evidence and the results obtained in each of the consulted works are presented.
| ID | Authorship | Title | Journal | Objective | Type of study/sample |
|---|---|---|---|---|---|
| 1 | Wang, et al. | Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China | The journal of infectious diseases | To present the epidemiological and clinical characteristics of patients with asymptomatic SARS-CoV-2 infection | Prospective cohort 55 patients |
| 2 | Ye, et al. | Clinical efficacy of lopinavir/ritonavir in the treatment of coronavirus disease 2019 |
European review for medical and pharmacological sciences | To evaluate the clinical effect of lopinavir/ritonavir combined with routine adjuvant therapeutic drugs in patients with COVID-19 | Case-control study 47 patients |
| 3 | Aghdam; Jafari; Eftekhari | Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report | Infectious disease | Report the case of a 15-day-old newborn diagnosed with the new coronavirus and with clinical signs of sepsis | Case study 1 newborn |
| 4 | Hrusak, et al. | Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment | European journal of cancer | Conduct an instant survey on the incidence and severity of COVID-19 among children undergoing antineoplastic treatment | Ecological study approximately 10,000 patients |
| 5 | Lin, et al. | The isolation period should be longer: Lesson from a child infected with SARS‐CoV‐2 in Chongqing, China | Pediatric pulmonology | Report the case of a 7-year-old child diagnosed with the new coronavirus | Case study 1 patient |
Table 1: Summarization of the studies selected for this review regarding authorship, title, journal, objective, type of study and sample.
| ID | Level of evidence | Degree of recommendation | Results |
|---|---|---|---|
| 1 | 2B | B | Lopinavir-ritonavir was offered to a 2-year-old child for 7 days |
| 2 | 3B | B | Lopinavir-ritonavir has resulted in good responses in remission of fever and ammunition. The blood biochemical test suggested that the number of patients with abnormal ALT and AST in the test group did not increase significantly with the duration of treatment and the corresponding percentage was lower than in the control group; in patients receiving the combined LVV/r treatment, accelerated remission may be seen in some clinical symptoms, such as abnormality of leukocytes, lymphocytes and C-reactive protein; The results showed that the abnormal proportion of leukocytes, lymphocytes, CRP and PLT in the test group was generally lower than in the control group after three treatments |
| 3 | 4 | C | Vancomycin, amikacin and oseltamivir: Recovery began gradually after the second day of admission. Respiratory distress and resolved spots. Oral feeding started and was tolerated. |
| 4 | 2C | B | Hydroxychloroquine, lopinavir ritonavir |
| 5 | 4 | C | Lianhuaqingwen: Chinese herbal medicine administered orally as a granule Nebulization by interferonα‐1b Oseltamivir: Oral ingestion may not reduce the replication of SARS-CoV‐2 |
Table 2: Summarization of the studies selected for this review regarding the level of evidence and results.
Most of the research (n=3) was carried out in China, initially the epicenter of the pandemic and as shown in Table 2, the majority (n=3) was classified with degree of recommendation B, from the quality level of the evaluated scientific evidence. Only one of the studies presented an expressive sample, with the participation of 10,000 patients, who were part of a multicenter study involving 25 countries. However, all selected articles presented theoretical foundation in the analysis and presentation of their results. As for the approach, quantitative (n=3) predominated, with different types of methodological design.
The results related to the objective of this review were summarized in Table 2, in which the level of evidence and degree of recommendation are also presented. The drugs mentioned in the treatment of children diagnosed with COVID-19 belong to the class of antivirals, antibiotics, antimalarials, immunomodulators and phytotherapics which were: Lopinavir-Ritonavir (LPV/r); oseltamivir; vancomycin; amikacin; hydroxychloroquine; interferon α-1b; and lianhuaqingwen.
It is important to highlight that although the selected articles presented the drugs administered to children with COVID-19, only one of the studies focused on evaluating the effectiveness of a specific drug (LPV/r) in the context of the new coronavirus. In three of these studies the outcomes related to the drugs mentioned are presented and two of the selected studies were only in the descriptive field, which was pointed out as a limitation of these studies, which addresses the recommendations made by the consulted authors and the limitations identified by the authors of this study.
The combined treatment LPV/r was the most cited and its use was pointed out as effective in remission of fever and ammunition. Thus, in a case-control study, results indicated that, in comparison with the control group, patients in the test group returned to normal body temperature in a shorter time (test group: 4.8 ± 1.94 days vs. control group: 7.3 ± 1.53 days, p=0.0364), with such results suggesting that patients treated with LPV/combined with adjuvant drugs associated with pneumonia were more likely to return to normal body temperature than those treated with adjuvant drugs only [2].
It was demonstrated that the combined LPV/r treatment is able to reduce the abnormal values of the biochemical indices to the detriment of the adjuvant therapy alone when it was observed that the abnormal proportion of leukocytes, lymphocytes, PCR and PLT in the test group was generally lower than in the control group after three treatments. In addition, the abnormal proportion of lymphocytes, Hb, granulocytes and CRP in the test group gradually decreased from the first to the third measurement. The number of days required for nCoV-RNA to become negative was shorter in the test group (7.8 ± 3.09 days) than in the control group (12.0 ± 0.82 days), with p=0.0219.
It was also identified that the combined use of LPV/r with adjuvant drugs does not represent adverse, toxic or side effects of the liver, since biochemical tests revealed that the abnormal percentage of ALT and AST in the test group was lower than in the group control.
Regarding antibiotics, although one of the studies recommended that they should not be used and if used, that it should not exceed the period of 5 days due to the risk of new infections, in another study the authors showed that the combined antibiotic and antiviral treatment was responsible for the gradual recovery of the neonate after the second day of admission, having resolved the spots and respiratory distress. In addition, oral feeding began and was tolerated [3].
Regarding oseltamivir, mentioned in two of the studies, it was observed that it may not reduce the replication of SARSCoV- 2. Furthermore, depending on the situation of the patients, glucocorticoids can be used in a short period of time (3-5 days) at doses not exceeding 1-2 mg/kg/d.
Since the new coronavirus was announced as a pandemic by the World Health Organization (WHO) in March 2020, several studies are being developed in order to present clinically proven pharmacological treatments for this problem.
Although there is no specific drug available against COVID-19, authors have already positioned themselves on a strategy known as “repositioning/reuse”, which is related to the discovery of the action of existing drugs for diseases in addition to those for which they were originally developed, which applies to the drugs discussed in this study.
As it is an RNA virus, just like HIV, the replication and viral assembly of the new coronavirus is associated with protease hydrolysis, so that it is possible to prevent viral replication by inhibiting this process. OLPV/af is a protein inhibitor and one of the drugs currently used in the second-line treatment of retroviruses and in the context of HIV, interferes with protease causing deregulation of structural and functional proteins in the virus nucleus, contributing to the generation of immature and non-infectious viral particles, managing, in turn, to inhibit the replication of the human immunodeficiency virus. Because of its potential to reduce the viral load of HIV positive patients, its efficiency has already been highlighted in different studies.
Regarding SARS-CoV-2, it was pointed out by research that addressed therapies targeting the new coronavirus that the non-structural proteins encoded by the genome of this virus are protagonists in the viral life cycle and that the foci of binding in the proteases SARS-CoV-2 can be used as potential antiviral targets. In view of these findings, authors recommend taking into account the potential of antiviral agents that have already been approved or are under development for the treatment of infections caused by HIV and other viral infections, among which LPV/r, which is one of the HIV protease inhibitors [4].
Several studies point to the effectiveness of the combination of these drugs in reducing viral load and improving immunity, even when this drug is used without the combination with other antivirals. In a meta-analysis carried out with the aim of evaluating the results of the efficacy and safety of LPV/r in HIV-infected individuals, the authors concluded that it is an effective therapy, with a significant effect, including in the prevention of vertical transmission. In another study which specifically dealt with HIV infection in children and adolescents, it was suggested that better responses were obtained when the protease inhibitor was included in the therapeutic regimen, which culminated in greater viral suppression by the treated patients.
Although there is already evidence that the use of LPV/r can significantly reduce the development of acute respiratory distress syndrome and mortality caused by SARS infection, there is still little production on these drugs in the treatment of SARS. CoV-2, since it is a recently discovered strain [5]. However, it is reported that studies have already pointed to the effectiveness of using lopinavir, LPV/r in reducing viral load and better results in patients with COVID-19, with a reduction in the need for invasive ventilation. Despite such evidence, the Brazilian Society of Pediatrics (SBP) does not recommend the use of these antiretroviral in children.
In relation to oseltamivir, indicated for the treatment of SRAG by influenza virus it acts by inhibiting viral replication by inhibiting the flu virus neuroaminidase enzyme, which is essential for both the entry of the virus into uninfected cells as for the release of viral particles and further expansion of the etiological agent in the body and should be used in flu-like syndromes in pediatrics at an early stage, preferably within the first 48 hours from the date of onset of symptoms, as it is considered that the early introduction of this drug is capable of reducing the duration of symptoms and complications of infection [6].
When positioning itself about this drug in the treatment of children suspected of having COVID-19, SBP recommends its empirical use, regardless of the severity of the case and the drug should be kept until the time of the PCR result for the coronavirus and if virology for influenza is positive.
Even though it is known that in viral infections, antibiotics are ineffective, in influenza syndromes, adjunct antibiotic treatment with antiviral is recommended in relation to COVID-19, in the clinical management protocol of COVID-19 in specialized care, published by the Ministry of Health (MS) the association of antibiotic therapy with antiviral treatment is recommended as a way to prevent or treat possible associated bacterial infection [7].
In a survey carried out in a pediatric hospital in Portugal with children and adolescents affected by influenza, the author identified that in 63% of the cases there was a prescription for antibiotic therapy, having considered such a high rate and warned about the need to introduce antibiotics only when bacterial overinfection is suspected. A similar result was obtained by a retrospective cohort conducted at a pediatric hospital in the United States, which revealed a high number of individuals with viral infection receiving antibiotics in parallel. Such findings corroborate the position of authors who, when dealing with viral infections in pediatrics, reveal that coronaviruses are one of the main etiologic agents, with the nasal mucosa inflammatory process being an important cause of obstruction of the paranasal sinuses and tube. Hearing, with the possibility of secondary bacterial infection, requiring antibiotic therapy in parallel to treatment with antivirals. In view of such evidence, SBP recommends the introduction of antibiotics when bacterial infection is suspected and individual therapy should be used [8].
Hydroxychloroquine, the antimalarial mentioned in this review was at the center of discussions about treatment for cases of the new coronavirus, having been highlighted in different studies in which it was considered one of the alternatives for COVID-19. Although there are in vitro studies that reveal the effectiveness of hydroxychloroquine in inhibiting viral replication there are also studies that point to the existence of important adverse effects when using this drug. In addition, in one of the studies in which a decrease in viral load was observed in patients, it is possible to highlight the existence of some biases such as the fact that hydroxychloroquine was used in association with azithromycin, the absence of a control group for comparison of findings and a small sample. In view of these findings, the Brazilian society of Immunology concluded that the recommendation for the use of this medication in COVID-19 is premature, requiring further research in this regard.
It should also be noted that the SBP (48) in a scientific opinion on the use of hydroxychloroquine for the treatment of COVID-19 in children and adolescents, discourages the use of this drug because it considers that there are no data to support the safety and efficacy of its administration in pediatric patients with COVID-19, regardless of the severity of the case [9].
Α interferon, the drug of choice for the treatment of children affected by the hepatitis B virus acts in the regulation of cell growth with antiviral, ant proliferative and immunomodulatory effects. Its use in cases of the new coronavirus has not yet been documented in the literature. However, in a study that sought to clarify the pulmonary manifestations related to its use, it was revealed that cases of pulmonary toxicity attributed to interferon α have been progressively reported, with clinical manifestations ranging from asymptomatic to severe responses in which there is a risk of death [10]. In view of such evidence and the deleterious effects of the new coronavirus on the pulmonary system are already known, the use of α interferon should be cautious and its administration in pediatrics is not recommended.
In China, a country that was the epicenter of the pandemic and the main source of the articles selected for this review, traditional medicine has been an important care alternative in different contexts of illnesses for millennia with phytotherapy being one of the possibilities COVID-19 treatment system. Therefore, the use of lianhuaqingwen was highlighted, which has already been the object of a study in a randomized clinical trial in the context of Chronic Obstructive Pulmonary Disease (COPD) having proved to be effective for the disease in question due to to its potential to decrease the release of inflammatory mediators and consequently, to reduce systemic and airway inflammation. In the treatment of influenza, it has been shown to have antiviral activity and relevant impacts on the immune system [11].
In the specific case of the new coronavirus, studies have also shown its effectiveness. Research that aimed to demonstrate the antiviral activity of this herbal medicine pointed to a significant inhibition of SARS-CoV-2, a significant reduction in pro-inflammatory cytokines and abnormal morphology of viral particles in cells, allowing the authors to conclude that lianhuaqingwen is one of the control strategies of COVID-19. These results coincided with those published in a randomized clinical trial that aimed to determine the safety and efficacy of the use of lianhuaqingwen in patients with COVID-19 and that presented significantly greater recovery in the test group, in addition to a shorter recovery time between patients who received this medication and improvement in computed tomographic manifestations, without serious adverse events being reported.
There are several ongoing studies evaluating the efficacy and safety of drugs of different classes for patients with COVID-19. It is necessary to conduct controlled or randomized studies with a high number of patients so that the evidence presented is, in fact, strong and capable of guiding clinical practice and decision-making [12].
Scientific evidence about pharmacological therapy for COVID-19 points to the use of different drug classes, with an emphasis on antivirals (here represented by the combination of LPV/r and oseltamivir) for the treatment of children with the new coronavirus. In addition to these, the use of antibiotics, antimalarials, immunomodulators and herbal medicines stands out.
Although all the drugs discussed have shown a positive aspect in the context of the treatment of children with COVID-19, it is premature to state that such drugs are the best option for pharmacological management of the new coronavirus in pediatrics and it is necessary to conduct further research on this thematic. Therefore, it is recommended to conduct clinical, controlled and randomized studies to support strong evidence, guiding the best evidence-based practice.
The limitations of this study include the scarcity of productions on the theme explored, especially in the national literature, and the fact that most studies selected for this survey have not discussed in depth the outcomes of the drugs used among children.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Siqueira SMC, Pereira SIV (2024) Scientific Evidence about Pharmacological Therapy for COVID-19 in Pediatrics. Qual Prim Care. 32:15.
Copyright: © 2024 Siqueira SMC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.