Research Article - (2023) Volume 9, Issue 2
Received: 29-Mar-2023, Manuscript No. IPBM-23-16207; Editor assigned: 31-Mar-2023, Pre QC No. IPBM-23-16207 (PQ); Reviewed: 14-Apr-2023, QC No. IPBM-23-16207; Revised: 19-Apr-2023, Manuscript No. IPBM-23-16207 (R); Published: 26-Apr-2023, DOI: 10.35841/2472-1646.23.09.011
Background: Autism Spectrum Disorder (ASD) is characterized by conditions involving areas of social interaction, communication and behavior, as well as sensory sensitivity. Studies have reported an association of ASD with alterations in protein and amino acid metabolism. The aim of this study was to identify the profile of proteins and amino acids in the urine of children with ASD.
Methods: Prospective cross-sectional study with case-control design. The cases were male children (n=22) with ASD, aged 3 to 10 years, and the control group was formed by neuro-typical children (n=22), matched for sex and age. The determination of the amount and composition of proteins was performed by the Bradford method and the determination of the amount and composition of amino acids by ultra-efficient liquid chromatography (CLUE).
Results: Alterations in protein and amino acid concentrations of arginine, glycine, leucine, threonine, aspartic acid, alanine, histidine, and tyrosine were identified in the urine of children with ASD. The abnormal levels of proteins and amino acids may be related to several symptoms observed in people with ASD.
Conclusion: The concentration of total protein and amino acid profile in urine are good candidates as biomarkers for individuals with ASD.
Autism spectrum disorder; Biomarkers; Urine; Protein; Amino acids; Metabolism
Autism Spectrum Disorder (ASD) is a clinical syndrome characterized by neurodevelopmental abnormalities in social and language interaction, repetitive or restricted patterns of interest, and changes in sensory sensitivity American Psychiatric Association [1]. Symptoms are heterogeneous and vary in the intensity from mild to very severe. In addition to the core symptoms of the disorder, severe conditions coexist with ASD. Intellectual disability is to present in varying degrees in approximately 30% to 50% of cases and more than half of this population suffers from a psychiatric disorder such as bipolar disorder, depression, anxiety, attention deficit hyperactivity disorder [2,3].
The prevalence has increased dramatically in the last two decades, with studies suggesting that the prevalence of ASD in children is greater than 1%. The male sex is more affected, with an average ratio of 4 to 5 males to 1 female [4-6]. Its etiology is not understood, but there is a consensus that the disorder is the result of a complex interaction between multiple and variable genes, epigenetic effects, and environmental factors. The diagnosis is made by clinical observation and there are no typical biomarkers or endophenics to identify and characterize the disorder [2,6].
The definition of ASD does not mention of the occurrence of metabolic changes, but this condition is being observed in approximately 5% to 30% of individuals with ASD [7]. In the mid- 1980s, studies reported changes in protein concentration as well as variations in the concentration of serotonin, catecholamines and opioid neurotransmitter concentrations in cerebrospinal fluid, plasma and urine in individuals with ASD. These findings have led to the development of the study are to investigate am alterations in the metabolism of protein and amino acids involved in neurotransmission. The results of published studies on the concentrations of these amino acids in the urine of individuals with ASD are diverter, and many theories have been developed from them [8,9].
For all that has been exposed, the objective of this work was to describe a metabolic profile from the comparison of the total protein and amino acid concentration between urine samples from children with ASD with urine samples from neurotypical children to verify the possibility for better characterization of the condition based on metabolic biochemical data.
The study involved 18 individuals (males and females) diagnosed with ASDs under the age range of 8-29 years recruited from Children Development Centre (CDC), Surulere, Lagos State and was conducted at the national stadium and gym of the centre. This study excluded individuals with comorbidities such as HbSS, Epilepsy, visual impairment or hearing deformity, and those who consistently engaged in dance therapy prior to the start of the intervention program [5].
All procedures were carried out in accordance with the National Health Council Resolution No. 466 (2012). The present project was presented to the Research Ethics Committee of the Faculty of Medicine of the University of São Paulo, in the meeting of 12/14/2011, and approved with protocol number 449/11. Written informed consent was obtained from the parent or legal guardian.
The screening of children with ASD was carried out at the Municipal Specialization Center for Autism, located in the city of Limeira-SP, and at the Association of Parents and Friends of Autism of Baixa Mogiana-Fonte Viva, located in the city of Mogi- Guaçu-SP. The participating children were first diagnosed by psychiatrists and pediatricians and referred to these institutions. There, the diagnosis was confirmed by a multidisciplinary team according to the criteria of the ICD 10. The control group was constituted by boys with typical development. The enrollment of participants in the control group was carried out in five schools (Children’s Center-Lucinda Tank Kühl, EMEIEF-Pastor Ismael Pereira do Lago, EMEIEF-Maria Aparecida Machado Julianelli, EMEIEF Prof. Noedir Tadeu Santini, EE Leontina Silva Busch) and one religious institution (Dispensário Madre Teresa de Calcutá) in the city of Limeira-SP.
Inclusion criteria for children with ASD were: Male sex, age between 3 and 10 years, and being treated in the participating centers. The exclusion criteria were: Not participating in the treatment at the selected institutions, being a supporter of any nutritional intervention, having celiac disease and allergy or intolerance to cow’s milk, and having a diagnosis of a disease that affects the metabolism and excretion of proteins and amino acids, such as liver and kidney disease.
The inclusion criteria for neurotypical children were: Typical development, be the sex male and possess equivalent age of the child with ASD in the survey. The exclusion criteria were: Being adept at any nutritional intervention, having neuropsychiatric disease, having celiac disease and cow’s milk allergy or intolerance, and having a diagnosed disease that compromises the metabolism and excretion of proteins such as liver and kidney disease.
Urine Collection
The procedure was done at home. The first urine of the day was collected and stored in a sterile universal collector. The samples were delivered to the receiving points, transported in a container with dry ice to the laboratory of Biochemistry and Biophysics Institute Butantan and placed at a temperature of -80°C. The time between collection and freezing of samples was less than 4 hours.
Sample Preparation
The samples were slowly thawed at a temperature of 4°C, homogenized (Centrifuge Sorva ll Super Speed RC2-B, Thermo Fisher Scientific, Waltham, MA, USA), filtered through a 45 μm PVDF filter (Merck Millipore S/A®, Cotia, SP, Brazil) and fractionated in volumes of 1.5 mL. The post new fractionation, were stored at a temperature of -80°C until the moment to the experiment.
Protein Quantification of Bradford Method
Total protein concentration was measured according to the Bradford method (1976). The Bio-Rad protein assay (Bio-Rad Laboratories, Richmond, CA, USA) was used for the analysis. Bovine serum albumin (BSA) (Sigma-Aldrich, São Paulo, SP, Brazil) was used as a reference standard. The concentration of the samples was estimated by comparison with a standard curve of BSA (1.0 mg/mL-0.8 mg/mL-0.6 mg/mL-0.4 mg/mL-0.2 mg/ mL-0.1 mg/mL-0.05 mg/mL), plotted and analyzed by linear regression. Reading was performed using a spectrophotometer at 595 nm.
Separation and Determination of the Amino Acid Composition by Ultra-Efficient Liquid Chromatography (CLUE)
For the analysis of the composition and quantification of amino acids, ethanol and acetonitrile purchased from JT Baker (Xalostoc, Mexico), triethylamine, phenylisothiocyanate, and trifluoroacetic acid TFA produced by Sigma-Aldrich (St. Louis, USA), 100% glacial acetic acid purchased from Merck (Darmstadt Germany), and EDTA solution (pH 8.0) and sodium acetate: Commercialized by VETEC (Duque Caxias, Rio de Janeiro, Brazil) were used.
In vials, 40 μl of sample and 30 μl of the derivatizing solution were added (70% v/v ethanol, 10% v/v Milli-Q water, 10% v/v triethylamine and 10% v/v phenylisothiocyanate), then homogenized and left to stand at room temperature for 15 minutes, then 170 μl of Milli-Q water was added. A volume of 50 μl was injected and analyzed in an ultra-efficient liquid chromatographysystem (CLUE) (LC20 AT, (Shimadzu®, Kyoto, Kinai, Japan). Column C 18 250 mm 4.6.7 μm was used for the analysis and a guard column of the same material. the mobile phase a consisted of 470 ml buffer solution+30 ml of ACN mobile phase B and 400 mL ACN+100 ul EDTA solution (pH 8.0). The solution buffer was prepared c with 19 g of sodium acetate+1000 ml Milli-Q water+0.5 ml triethylamine+200 μL EDTA solution (pH 8.0). The elution gradient had a flow rate of 0.3 ml. And the mobile phase gradiente protocol as follows: 0 to 24 minutes 46% of phase a 24 to 30 minutes at phase a has increased its concentration to 100%, the system maintained balanced with phase A at 100% for 8 minutes. The total analysis time was 47 minutes. The reading was performed with a PDAUV-Vis detector at a wavelength of 254 nm.
Amino acid quantification was determined based on the retention time and peak area obtained from the known concentrations of the amino acids aspartic acid, glutamic acid, serine, glycine, histidine, arginine, threonine, alanine, proline, tyrosine, valine, methionine, cysteine, isoleucine, leucine, phenylalanine and lysine.
Statistical Analysis
The information was entered into an electronic spreadsheet using the Microsoft® Excel program. Analysis statistics were performed using the Bioestat program, version 5.3 (Mamirauá Sustainable Development Institute). Protein and amino acid concentrations were expressed as median and interquartile range. The Shapiro-Wilk test was used to check the normality of the data, and then the Wilcoxon-Man-Whitney test was used to analyze the difference between the medians of the concentrations of the compounds analyzed.
A comparative analysis between the median protein concentrations of the urine samples (mg/ml) of the participating groups shows a statistical difference between children with ASD and neurotypical children. The protein concentration in the urine samples of the neurotypical group is significantly higher than that observed in children with ASD. In the amino acid profile analysis, differences were found between the groups of participants for eight amino acids. Urine samples from children with ASD showed higher concentrations of the amino acids arginine, glycine, leucine, and threonine than samples from the control group. The concentration of the amino acids aspartic acid, alanine, histidine and tyrosine were lower in urine samples from children with ASD than in urine samples from neurotypical children (Table 1). Summarizes the results of the analyses. Illustrates the chromatographic profile of urine from child with ASD and neurotypical child (Figures 1 and 2).
Table 1: Result of the separation and determination of the amino acid composition by ultra-efficient liquid chromatography (CLUE).
| Amino Acids | Descriptive statistics | Wilcoxon-Mann-Whitney test | ||
|---|---|---|---|---|
| Children with ASD | Neurotypic children | U value | P value | |
| Median (IQR) (mg/ml) | Median (IQR) (mg/ml) | |||
| Total Protein* | 7.95 (6.30-12.60) | 24.00 (13.25-53.35) | 114.5 | 0.0014 |
| Aspartic acid* | 0.004 (0.002-0.009) | 0.025 (0.015-0.037) | 71 | <0.0001 |
| Glutamic acid | 0.009 (0.003-0.018) | 0.010 (0.001-0.026) | 239 | 0.71 |
| Alanine* | 0.016 (0.008-0.037) | 0.047 (0.029-0.065) | 140 | 0.008 |
| Arginine* | 0.025 (0.011-0.036) | 0.000 (0.000-0.058) | 168 | 0.041 |
| Cysteine | 0.004 (0.003-0.007) | 0.001 (0.000-0.026) | 186 | 0.094 |
| Phenylalanine | 0.010 (0.006-0.017) | 0.013 (0.007-0.025) | 195 | 0.135 |
| Glycine* | 0.038 (0.017-0.098) | 0.026 (0.019-0.035) | 167 | 0.039 |
| Histidine* | 0.004 (0.003-0.011) | 0.101 (0.027-0.134) | 72 | <0.0001 |
| Isoleucine | 0.003 (0.002-0.005) | 0.013 (0.000-0.016) | 192 | 0.12 |
| Leucine* | 0.024 (0.015-0.047) | 0.018 (0.006-0.030) | 168 | 0.041 |
| Lysine | 0.017 (0.008-0.028) | 0.013 (0.007-0.020) | 220 | 0.302 |
| Methionine | 0.002 (0.001-0.004) | 0.000 (0.000-0.020) | 218 | 0.286 |
| Proline | 1,226 (0.848-1,502) | 1,192 (0.532-1.431) | 205 | 0.192 |
| Serina | 0.058 (0.037-0.078) | 0.052 (0.029-0.096) | 239 | 0.471 |
| Tyrosine* | 0.017 (0.009-0.028) | 0.042 (0.021-0.066) | 127 | 0.0035 |
| Threonine* | 0.025 (0.009-0.0659) | 0.000 (0.000-0.018) | 90 | 0.0002 |
| Notes-IQR=interquartile range. *compounds with significant P value<0.05 |
||||
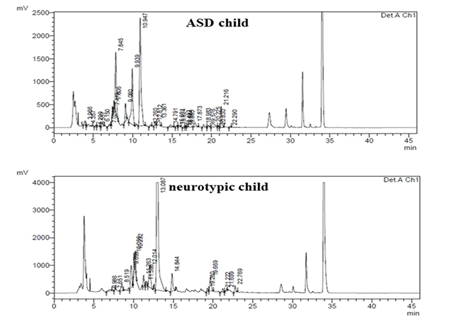
Figure 1: Chromatogram of the childâ??s amino acid profile with ASD and their respective control. Detection at 254 nm. Mobile phase A (470 ml buffer solution+30 ml ACN) and mobile phase B (400 ml ACN+100 µl EDTA solution at pH 8.0).
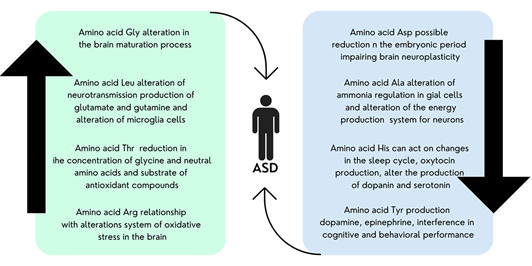
Figure 2: Illustration of the relationship of possible metabolic alterations of the amino acids arginine, glycine, leucine, threonine, aspartic acid, alanine, histidine and tyrosine with symptoms of ASD.
In our work, nine compounds showed differences between ASD children and the control group, suggesting that differences in the excretion of some amino acids could be related to some symptoms of the syndrome as described in the literature.
The protein concentration of the group of children with ASD was statistically lower than that of the control group. This finding may be related to the fact that a large proportion of the population of children with ASD has a restricted diet. Studies have shown a direct correlation between a diet low in high biological value proteins and Vitamin B and low levels of amino acids in the body fluids of children with ASD [10,11].
There are few published studies on the adequacy of nutrient intake in individuals with ASD or research that compared intake with a control group. The results of the studies are conflicting and this makes it difficult to define the eating pattern or a tendency to consume foods specific to the disorder. One of the reasons for the inability to define an eating pattern for individuals with ASD is related to the high prevalence (30% to 90%) of inappropriate behaviors associated with eating [12-14].
Behavioral abnormalities related to eating are likely to be associated with central deficits in ASD. The deficit in social interaction, coupled with repetitive behaviors, restricted interest, and lack of appropriate behavioral models for eating, can impede the learning of children with ASD in various activities, such as eating with appropriate utensils. Many individuals with ASD do not develop communication skills. This fact prevents them from expressing their needs such as hunger, fullness, food preferences, and discomfort after meals [15-17].
Another fact that also limits food consumption is the high prevalence of sensory symptoms in people with ASD (from 69% to 93%) [18-21]. The main problems of sensory modulation are expressed as hypersensitivity and/or hyposensitivity. Such problems have an impact on the individual’s development and the ability to perform activities of daily living such as eating [20,22- 26].
It cannot be ruled out that the low protein concentration in urine samples from children with ASD may be related to inadequate digestion of dietary protein. There is no direct evidence for this hypothesis, but some research supports it. Study reports describe reduced activity of several saccharolytic digestive enzymes and a possible irregular secretin response in the pancreas of individuals with ASD [27]. Inadequate digestion of proteins and their derivatives may also manifest as a phenotype of altered concentrations of specific amino acids in body fluids [9,10,28].
In our work, the result of the amino acid profile analysis shows a high concentration of the amino acids arginine, glycine, leucine, and threonine in urine samples from children with ASD compared to urine samples from the control group.
Arginine is a semi-essential proteinogenic amino acid that is widely distributed throughout the central nervous system (CNS) and is involved in the production of several bioactive molecules, including nitric oxide (NO), L-citrulline, L-ornithine, urea, and agmatine [29-32]. Arginine and NO are involved in learning and memory functions, synaptic plasticity, neuroprotection and neurogenesis [33-36].
Excess arginine is thought to induce oxidative stress via NO production [37]. High levels of NO have been reported in the plasma of children with ASD [38]. Studies on the concentration of arginine in body fluids of individuals with ASD have shown controversial results [39-43]. Research has shown an association between neuropsychiatric disorders and arginine concentrations in body fluids. In a postmortem study, Liu et al. (2016) found altered concentrations of arginine and its major metabolites L-citrulline, L-ornithine, and agmatine in the gray matter of the left frontal cortex of schizophrenic patients compared to a control group [44]. The precise nature of the relationship between arginine and oxidative stress in neuropsychiatric disorders is unclear; however, the common susceptibility genes for ASD and schizophrenia, TCF4 and NOS1, have been implicated in the arginine-NO pathway [45,46].
Glycine acts as a post-synaptic inhibitory transmitter, predominantly in the spinal cord and brainstem, and as a co-agonist of the glutamate-activated ionotropic receptor (NMDA), modulating its activity particularly in the cortex and forebrain. This amino acid is involved in locomotion, hearing and cognitive functions that may be important during the rapid growth period of the brain [42,47-50]. During embryonic and perinatal development, spontaneous transients play an essential role in various neuronal developmental processes such as neuronal migration, myelination, cortical regionalization or establishment of neuronal connectivity in various structures such as retina, spinal cord, auditory system, trigeminal system and cortex [51-59]. Rhythmic activities are influenced by gamma-aminobutyric acid (GABA) and glycine. They act as neutral excitatory messengers in the rhythmic activities of the immature brain, but failure of GABA and glycine to switch from excitatory to inhibitory neurotransmitters during birth and maturation can result in neural disorders, including ASD. The excess of glycine found in the body fluids of individuals with ASD may be a biological indicator of dysfunction in this signaling system that affects neuronal development [60,61].
Studies of plasma and serum samples from individuals with ASD have reported unchanged glycine levels [62,63]. The result of our research is similar to that of Mavel et al. (2013), Nadal-Desbarats et al. (2014), Noto et al. (2014) and Lussu et al. (2017), who found a high concentration of glycine in urine samples from individuals with ASD [41,64-66].
Elevated threonine levels in body fluids can alter the concentration of amino acids in the CNS. High threonine consumption leads to an increase in several non-essential amino acids, especially glycine and serine in the CNS [33,67,68]. Studies suggest that high plasma threonine concentrations may reduce the entry of other neutral amino acids by competing with transport systems in the blood-brain barrier [69,70]. Some neutral amino acids play an important role in the functioning of the CNS, as they participate in the production of neurotransmitters and also act as substrates for the production of antioxidant compounds such as glutathione and S-adenosylmethionine. Several studies have reported alterations in the redox system and also in the production of neurotransmitters derived from neutral amino acids in the CNS of individuals with ASD [71-73]. Previous studies that have analyzed plasma and urine from individuals with ASD have found results that are contrary to our work [63,74,75].
Leucine plays two important roles in the CNS: It participates in the regulation of the mammalian rapamycin (mTOR) signaling pathway and allosterically regulates the activity of some enzymes. It can contribute to the production of glutamate and glutamine by donating an amino group in the transamination reaction [76,77].
This amino acid, together with the other BCAAs, has the ability to modulate peripheral immune cells as well as microglial cells, but the excess of these amino acids impairs the neuroprotective functions in microglial cells, represented by the decrease in the production of the neuroprotective protein insulin-like growth factor type 1 (IGF-1). This effect may be related to the potential deleterious effects of mTOR hyperactivation [78]. Some research reports changes in microglial activation in the brain of individuals with ASD [79,80]. The high concentration of leucine in urine and cerebrospinal fluid samples from individuals with ASD has also been reported in several studies [10,63,74,81,82]. A recent study by Smith et al. (2019) identified potential metabolic dysregulation between the amino acids glycine, glutamine, ornithine, and BCAAs in 16.7% of children with ASD studied [83].
The concentration of the amino acids aspartic acid, alanine, histidine and tyrosine was lower in the urine samples from the group of children with ASD when compared to the urine sample from the group of neurotypical children.
Aspartic acid (C4H7NO4) has excitatory effects on the CNS, being particularly concentrated in the hippocampus and hypothalamus. It is present in the mammalian brain and participates in the regulation of plasticity itself náptica [84-86]. The concentration of aspartic acid is high throughout the brain during the embryonic and perinatal period and decreases sharply during adulthood [84,85,87-89]. From these statements, we can speculate that the low concentration of aspartic acid in body fluids may be the manifestation of inadequate concentrations during uterine development, which would cause changes in synaptic plasticity observed in individuals with ASD [90,91]. The result of our work corroborates previous work that identified low aspartic acid concentrations in urine and platelet samples from individuals with ASD [43,74,92].
Alanine does not readily cross the blood-brain barrier, but is generated within the CNS, particularly in astrocytes, by the pyruvate transamination reaction catalyzed by the enzyme alanine transaminase. The glutamate-glutamine cycle important for brain function is incomplete without the return of ammonia to glial cells. Alanine is involved in this cycle as an important transporter for the transfer of ammonia [93].
Alanine, like glutamate and glutamine, can be used as a substrate for gluconeogenesis and glycogen synthesis in cultured astrocytes and brain tissue. The low concentration of this amino acid in body fluids may reflect an altered concentration in the CNS and thus affect the energy metabolism of neuronal cells. Mitochondrial dysfunction has been identified in individuals with ASD, but due to the lack of studies, the concentration of alanine in body fluids is not considered a reliable biomarker for this condition [12]. The low concentration of the amino acid alanine in urine samples from children with ASD, when compared to samples from neurotypical children identified in our study, was also observed in the study by Evans et al. (2008) and Ming et al. (2012) [74,94].
The amino acid histidine is a precursor of histamine. This neurotransmitter is released by mast cells, basophils and neurons [95-100]. Histaminergic neurons originate from the tuberomamilar nucleus of the posterior hypothalamus and send projections to most of the brain [96]. All available evidence from different species indicates that histaminergic neurons are important for the regulation of sleep and wake cycles [101]. Based on all of the above, we can speculate that the low concentration of histidine in body fluids may be related to the fact that, on average, 50% to 80% of children with ASD are affected by sleep disturbances [102].
This neurotransmitter is also a regulator of many hypothalamic functions. Neuroendocrine responses, especially the release of vasopressin arginine, are physiologically regulated by histaminergic neurons [103,104]. Hypothalamic histamine may also be involved in the physiological regulation of oxytocin, prolactin, adrenocorticotropic hormone (ACTH) and β-endorphin release. The reduced concentration of histidine may be related to the low plasma concentration of oxytocin in individuals with ASD, as well as the arginine neuropeptide in vasopressin [105-107].
Histidine also acts as an inhibitor of neurotransmitter release, and its deficiency may contribute to hyperactivation of the CNS. These data may be related to the results of some studies that reported alterations in the production of serotonin, dopamine in individuals with ASD [108-113].
Some research has shown that alterations in the histaminergic system are present in neurodegenerative diseases and psychiatric disorders [114,115]. Cerebral histamine levels are reduced in patients with Alzheimer’s disease [114]. Research suggests that low levels of histamine in body fluids are associated with the occurrence of seizures [116-118]. On average, epilepsy affects 5% to 46% of individuals with ASD [119-121]. The low level of histamine in urine samples from children with ASD found in our study was also reported in the study by Evans et al. (2008), Ming et al. (2012) and Nadal-Desbarats et al. (2014) [64,74,94].
Tyrosine is a substrate for the production of catecholamines (dopamine, norepinephrine, epinephrine) [122-125]. Research indicates that low levels of tyrosine lead to reduced neurotransmitter availability and decreased cognitive and behavioral performance [125-127]. The concentration of this amino acid in the brain is directly influenced by the protein consumed not only in a single meal, but also by the protein content profile of the diet over several days [128-133].
The low concentration of this amino acid may be related to the lower protein consumption or insufficient metabolism described at the beginning of our discussion. The result of our work adds to the results observed in the literature [43,63,74,82]. After discussing possible mechanisms, we can hypothesize that dysfunctions in protein and amino acid metabolism may operate in several ways in the pathophysiology of ASD.
After discussing possible mechanisms, we can hypothesize that dysfunction in protein and amino acid metabolism may act in several ways in the pathophysiology of ASD: One way would be during the fetal and postnatal period when neurotransmitter receptors are undergoing functional development, making the brain selectively immature and vulnerable to overstimulation by neurotransmitter receptors. Deregulation of amino acid concentrations may also contribute to the exacerbation of core symptoms of the disorder and comorbidities.
Implication
Currently, there is no strong evidence that any single metabolite is significant for detecting or conclusive for identifying the etiology or pathophysiology of ASD. There is consensus that biomarkers may be used in the future to identify subgroups of individuals with ASD who have metabolic dysfunction. Several urinary metabolites are potentially useful, but controlled studies with larger numbers of participants are needed to validate them. Total urinary protein concentration and amino acid profile were shown to be good candidates for biomarkers or management of individuals with ASD; these data were an exciting starting point for population-based studies that will provide new information for clinical use Endnotes.
This article is part of the Doctoral Thesis entitled “Identification of Candidates for Urinary Biomarkers for Autism Spectrum Disorder,” submitted to the Postgraduate Program in Sciences of the Disease Control Coordination of the Ministry of Health of the State of São Paulo, to obtain the title of Doctor of Science. Author Dr. N. I. S. Advisor Dr. I. L.
Coordinator: I. L.; Conception and design: N. I.S., A.A.C., I. L.; Collection and assembly of data: N. I.S., A.A.C., I. L.; Data analysis and interpretation: N. I.S, A.A.C., I. L.; Manuscript writing: N. I.S., I. L. Final approval of manuscript: All authors.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
The datasets generated during and analysed during the current study are available in the Mendeley Data and Digital Commons Data repository, DOI:10.17632/78ygyh8sb6.2
We thank all the institutions that allowed us to recruit volunteers. We also thank the parents of children with ASD and neurotypical children who agreed to their children’s participation in the to participate in the research project.
All authors declare that they have no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Schoalar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Silva NI, Cunha AA, Lebrun I (2023) Searching for Biomarkers Candidates of Autism Spectrum Disorder with Metabolic Disorders Evidences for a Possible Role of Proteins and Amino Acids Content in Urine. Biomark J. 9:011.
Copyright: © 2023 Silva NI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.