- (2008) Volume 9, Issue 4
Ake Andren-Sandberg1, Philip D Hardt2
1Department of Surgery, Karolinska University Hospital. Huddige, Sweden
2Third Medical Department, Giessen and Marburg University Hospital. Giessen, Germany
Autoimmunity; Congresses as Topic; Diabetes Mellitus; Islets of Langerhans; Pancreas, Exocrine; Pancreatitis
CEL: carboxyl-ester lipase; DPP-4: dipeptidyl peptidase 4; ECM: extracellular matrix; GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagonlike peptide-1; IGT: impaired glucose tolerance; NIDDM: non-insulin dependent diabetes mellitus; PanIN: pancreatic intraductal neoplasia; PP: pancreatic polypeptide; PSC: pancreatic stellate cells; VNTR: variable number of tandem repeats
The ‘Second Giessen International Workshop on Interactions of Exocrine and Endocrine Pancreatic Diseases’ was organized as a followed-up of the first meeting that had been held in the castle of Rauischholzhausen, Justus-Liebig-University of Giessen, in 2005 (Proceedings published in JOP. Journal of the Pancreas (Online) [1]). Just as last time the meeting was organized in the perfect surrounding in Castle of Rauischholzhausen outside Giessen (Figure 1), which gave the 30 invited delegates - all very able and prone to discuss and comment on everything that was lectured about - a possibility to concentrate on the issues presented under pleasant forms, and gave good possibilities also to discuss outside the conference table (Figure 2).
The castle of Rauischholzhausen was first mentioned in a charter book of the monastery of Fulda in the second part of the 700s and was initially a fief of the Lords of Eppstein until the Archbishop of Mainz acquired it completely in 1369. From then on the vassals called themselves Lords Rau of Folzhausen, one of the knights on the eastern bank of the Rhein. The last member of the Rau family served as an officer in the Hessian army. When Hesse-Kassel became part of Prussia he refused to join the Prussian army and sold all his property to the ambassador’s delegate Stumm. The new owner was an industrialist that was ennobled by Kaiser Friedrich in 1888. After the war, in 1945, the castle and park were put at the disposal of the Justus Liebig University Giessen as a conference center. The park is designed in the English style and contains almost 300 different types of trees.
In his welcome adress Philip D Hardt (Giessen, Germany) pointed out that type 3 diabetes has gained attention by the international community and that the Giessen group organized a session on the topic at the CODHy meeting in Berlin in October 2006 (www.codhy.com) attended by more than 1,700 participants. In the meantime it has been accepted by most diabetologists that exocrine dysfunction is frequent in diabetes mellitus. There have been studies by different groups performing fecal elastase 1 measurements in diabetic patients, some of them adding new, interesting aspects. The most probable explanation for exocrine and endocrine co-morbidity is that type 3 diabetes mellitus must be a common disease, rather than a rare one. Further progress has been made in genetic research, since a mutation was found in the CEL gene causing exocrine fibrosis and diabetes. Another interesting aspect (pathologic changes of the incretin axis in patients with exocrine insufficiency and steatorrhea) is currently under investigation. Concerning chronic pancreatitis, paradigms have been started to be evolved. Pancreatitis or pancreatic fibrosis appears to be frequent in Western societies and might affect up to 10% in population based studies. Alcohol consumption seems to be only a minor pathogenetic factor, while autoimmunity, genetic defects and chronic obstruction have received more attraction recently.
Progress, Cessation and Dogmas in Medical Science
The meeting was started by Jürgen Hardt (Wetzlar, Germany), president of the Hessian Chamber of Psychotherapists, who gave a lecture on scientific progress. He stated that established research programs usually do not care about their scientific status; on the contrary, doing their scientific work there is nearly no time to reflect what the scientific status of their endeavor is. Only in the beginning or in times of crisis scientists are forced to think about the basis of their doing. Talking about scientific progress needs some clarification what is to be understood using the term science:
- science as a cultural institution, a social project with more or less autonomy;
- science as the occupation of a group of professionals, a subgroup of the scientific community;
- science as the performance of a single individual, a scientist, but a member of a scientific community.
Nowadays it is taken for granted that science is more or less autonomous. Highly specialized societies obliged to freedom of thinking offer the means to pursue scientific projects in order to cope with problems or just to find out what is true. Maybe because of taking it for a kind of natural thing scientists are not suspicious enough about attacks on scientific autonomy. There are indications that society is less and less willing to finance autonomous scientific project.
Medicine seems to be in an advantageous position but the plans of reorganization are ready. What the future of a reorganized university that produces education for social exploitation will be can be observed in the field of health economy. There the so called reform processes produced an economic and administrative dominance over therapeutic expertise. Therapists as lobbyists of the so called “Lebenswelt” (that is the world in which we really live) are nearly impotent in face of an economy and an administration.
In the second part of the 20th century theory of science (as a conception how science should proceed in order to reach truth) had been completed by historical studies - how scientists really did their job. This more realistic view of science led to a softening of the idealized norms of the scientific process. It was then discovered as to be very much more irregular as many authorities had seen and taught it to be. Science is not progressing in a linear way on the contrary the way of science is unforeseeable, there are curves, turn-offs, dead ends, main and side tracks, heavily traveled thoroughfares where nothing new can be discovered. The picture one gets when realizing how science really progresses might confer an impression of “anything goes”.
‘Anything goes’ was meant by Paul Feyerabend as a proclamation against the theory of Karl Popper. Popper unified the diverse scientific processes in a modernistic way. His critics were therefore disqualified as purely post modernistic protesters.
Thomas Kuhn’s ‘Structure of Scientific Revolution’ discerned two kinds of scientific progress: the slow, steady and industrious progress of so-called normal science and the sudden change of basic scientific concepts.
Normal science is bound to knowledge of tradition, mastering of familiar techniques, knowing the actual literature and so on. Normal scientists exactly know which questions are at stake and where and how solutions are to be found. Progress in normal science is to be discerned from sudden changes when basic concepts need to be changed. That is what Thomas Kuhn called a scientific revolution. It is a break with tradition and the formulation of a new basis of understanding. After a revolution science comes back to its normal processing. The difference between the two processes were grossly simplified, though Kuhn protested against the misreading; the “scientific revolution” hit a cultural movement, the students’ revolution.
Descartes had a traditional education and is known as the father of modern, antischolastic science. He advocated a scientific process of doubting like scholastic discussing and defending, but more radical and not distributed to discussing partners. His scientific doubting was an internal process. He aimed to find a firm basis that could serve as a fundamentum inconcussum, in order to rebuild a new and true world. He found certainty in him alone. “I doubt; therefore I am a doubting thing.” More familiar expressed: “I think therefore I am”. Descartes’ scientific work was moved by the tension between tradition and revolution.
A single scientist often aims at a kind of breakthrough; he seeks for a revolutionary idea and not for continuous normal work. But science progresses only in the tension between a tradition that serves as an unquestioned dogma and a state of successful revolution. The prerequisite of real progress seems to be not only to perform the next step but to risk a sort of breakthrough at the same time. The solution of standing in and stepping out of traditional ways of thinking is the secret of real scientific progress. Stepping out is much more than denying or negating his or her position in the flow of generations. What is needed is a sort of creative synthesis of the antagonistic positions.
Kuhn was deeply concerned about this problem. He thought about the question what the relation of an encompassing process of science and the work of a single creative scientist might be. “In one sense the procreating organisms, which perpetuate a species, are the units whose practice permits evolution to occur. But to understand the outcome of that process, one must see the evolutionary unit (...) as the gene pool shared by those organisms, the organisms which carry the gene pool serving only as the parts which, through sexual reproduction, exchange genes within the population. Cognitive evolution depends, similarly, upon the exchange, through discourse, of statements within a community. Though the units which exchange those statements are scientists, understanding the advance of knowledge, the outcome of their practice, depends on seeing them as atoms constitutive of a larger whole, the community of practitioners of some s cientific specialty.”
Session A: Pancreatic Diabetes (Type 3c)
Exocrine Function in Diabetes
Franco Cavalot (Turin, Italy) discussed exocrine pancreatic function in established diabetes mellitus. The pancreas is a complex, integrated organ that consists of three physically, structurally and functionally related components: the endocrine part, the exocrine acinar part, and the exocrine ductal part. Acinar secretion is regulated in a paracrine and endocrine way by islet hormones. There is a portal insulo-acinar system. About 15% of the pancreatic blood flow goes to the insulae, even though they are only 1-2% of the whole glad from a weight point of view [2]. This gives an endocrine/exocrine blood flow rate/weight as 12.
There are also isolated endocrine cells in pancreas (cells not connected to the insulae) [3]. Islet cells are of all types and seem to be mixed firstly with ductal cells. They have an apical pole with microvilli, smooth and coated vescicles (to exchange activity with ductal lumen). Some cells have many secretory granules at the apical pole. In man, this insulin-secreting cell population associated with the ductal system counts for 15% of the total beta-cell mass. However, there are also buds of endocrine cells outside the insulae. In the adult rat pancreas buds of endocrine cells, usually in clusters of 20-25, close to the intercalar and intra-lobular ducts have been found. They have not yet been found in man, which might be due to technical problems. Regarding the islets of Langerhans, it has been shown that about 75% of islets have multiple connections with ductal cells. The cells mainly involved are the glucagon and somatostatin secreting cells, located at the periphery of the islets. In some cases the ducts enter the islet and take close contact with the beta-cells. Thus, already on an anatomical level the endocrine and exocrine systems have deep interrelationships.
The islet cell hormones have profound effects on exocrine pancreas. Insulin and pancreatic polypeptide (PP) are trophic hormones. Insulin stimulates protein synthesis and cell division in acinar tissue [4]. PP increases DNA synthesis in rat pancreatic cells [5]. Glucagon and somatostatin on the other hand inhibit exocrine function [4] and prolonged parenteral administration of glucagon to rats causes pancreatic acinar atrophy [6].
In vivo insulin affects the exocrine pancreas in several ways:
- regulation of amylase content and amylase mRNA;
- increased protein synthesis;
- potentiation of CCK-induced secretion;
- regulation of CCK receptors;
- downregulation of acinar cells insulin receptors.
In vitro experiments have shown:
- increased glucose transport in isolated acini;
- increased protein synthesis in isolated acini;
- potentiation of CCK-induced secretion (perfused pancreas and isolated acini);
- downregulation of acinar cells insulin receptors;
- increased binding of IGF-II.
There is a positive effect of insulin treatment on amylase RNA in streptozotocin-induced diabetic rats [7]. The concentrations of insulin reaching the exocrine pancreas are much higher than those reaching the systemic circulation. Thus, the endocrine pancreas has profound paracrine effects on pancreas’ exocrine structures. There are also some interesting morphological findings in the exocrine pancreas type 1 diabetes:
i) in patients with long-lasting diabetes:
- reduced weight of pancreas;
- histological evidence of exocrine pancreas atrophy;
- 2/3 reduction in the weight of the glucagonrich lobe, but no atrophy in PP-rich lobe in spite of loss of beta-cells [8, 9, 10, 11];
ii) in patients with recent diabetes diagnosis (within 24 h):
- severe acinar atrophy in tissue surrounding insulin-deficient, glucagon-rich islets, but normal acinar tissue around insulincontaining cells [12].
According to Hardt et al. [13], the exocrine function in type 1 and type 2 diabetes mellitus shows high prevalence of pathological exocrine function in both type 1 and type 2 diabetic patients. There are lower levels of fecal elastase-1 in type 1 versus type 2 diabetic patients, and lower levels of fecal elastase-1 in insulin-treated patients. There is a weak association between fecal elastase and diabetes duration, age at onset of diabetes and body mass index. In type 1 diabetes fecal elastase-1 was not significantly related to BMI, daily insulin dose, insulin dose per kg body weight, caloric intake, urea nitrogen, and urinary albumin excretion [14]. However, elastase-1 is lower compared to non diabetic subjects and exocrine function is frequently compromised. Fecal elastase-1 positively correlates with residual beta-cell function and degree of metabolic control. The effect of diabetes duration on elastase-1 appears to be mediated by residual beta-cell function. Also in patients with compromised beta-cell function, residual insulin-secretion plays a role in modulating exocrine pancreas function.
In type 2 diabetes it has been found [15]:
- reduced levels of fecal elastase in type 2 diabetes as compared to normal controls;
- severe exocrine pancreas impairment in 12%, moderate and severe impairment in 30% of patients;
- insufficiency more frequent in males, even after correcting for strong alcohol consumption;
- significant influence of blood glucose control.
In a multicenter study steatorrhea in 101 type 1 and type 2 diabetic patients with severe exocrine pancreatic insufficiency (fecal elastase-1 less than 100 μg/g) was studied [16]. A high frequency of pathological fat excretion was reported in diabetic subjects with low (less than 100 μg/g) fecal elastase-1 with a coefficient of fat absorption less than 90% (i.e. fat maldigestion) in 20% of the patients. The authors concluded that exocrine pancreatic disease is more common than previously believed both in diabetic and non diabetic people, in agreement with autopsy studies that indicate pancreatic involvement in 13% of a “normal” population.
Fecal elastase-1 and steatorrhea has also been studied in type 1 diabetes [17]. It was found that among the type 1 diabetic patients almost one fourth have fecal elastase-1 levels below the normal range. Steatorrhea is frequent (29%), and is very frequent (70%) in subjects with fecal elastase-1 less than 100 μg/g stools. A high percentage (58%) of subjects with steatorrhea show normal fecal elastase-1 values, opening questions on the pathogenesis of the increased fat excretion. Treatment with pancreatic enzymes corrects the steatorrhea in subjects with low fecal elastase-1.
Finally, Cavalot proposed several different mechanisms for impaired exocrine function in diabetes:
- insulin deficiency -> defective trophic action of insulin;
- hormone suppression: high glucagon, or somatostatin, or both, levels;
- common autoimmune process;
- diabetic microangiopathy leading to pancreatic fibrosis;
- diabetic autonomic neuropathy and impaired enteropancreatic reflexes;
- primary pancreatic disease;
- mutations in HNF1B in MODY 5 and other monogenic disorders.
The Relationship between Diabetes, Celiac Disease and the Exocrine Pancreas
John S Leeds (Sheffield, UK) started by saying that the associations between diabetes and the GI tract are well known since at least 1936 [18], and it was discussed as “diabetic diarrhea”. Celiac disease is more common in male, type 1 patients of increasing duration, but no large prevalence studies in patients with type 1 diabetes mellitus [19]. Regarding diabetes it is well established that intensive glycemic control is the cornerstone of management that leads to reduced morbidity and mortality [20, 21]. However, other medical conditions may also contribute [22]. In a previous study [19] a postal survey of 15,000 persons with type 1 diabetes was made. There was a 60% response rate, but only 27 subjects had type 1 diabetes. The symptoms and glycemic control were self reported and no investigations were performed. In these patients an autonomic dysfunction and an altered enterohumeral responses was found.
There are many interesting areas with respect to diabetes and chronic GI disease, e.g. celiac disease, small bowel bacterial overgrowth, exocrine pancreatic insufficiency, thyroid dysfunction with GI symptoms, autonomic neuropathy, and maybe inflammatory bowel disease. Regarding celiac disease it is known [23] that an association between type 1 diabetes and celiac disease is recognized as well as an association of HLA markers or a Tcell mediated response. There is a large number of studies in diabetes cohorts looking for celiac disease and lots of pediatric literature on the possible benefit of screening shown. The prevalence ranges from 0 to 16.4% (n=47-4,154), but there are only 4 adult studies with at least 500 patients. The serological assays and glycemic markers differ and there has been a lack of control populations. The effect on glycemic control uncertain and the effect upon quality of life not assessed.
Mr Leeds and co-workers have investigated a large cohort of type 1 diabetes for GI symptoms, clinical correlations and underlying causes. Moreover, they have investigated a large cohort of coeliac patients for prevalence of celiac disease and have assessed pancreatic function. So far the preliminary results are based on 371 patients with type 1 diabetes mellitus and 603 healthy volunteers. Of the diabetics 20.6% had GI symptoms compared to 10.4% of the controls (odds ratio: 2.2; 95% confidence interval: 1.5 to 3.2), which was a highly significant difference. Of certain interest is that the difference among other symptoms consisted of weight loss, floating stools, diarrhea, and abdominal pain (i.e. symptoms one sees in chronic pancreatitis).
Looking the other way around 1,000 patients with celiac disease were recruited from 1,043 approached (96% uptake) and investigated for diabetes. The known prevalence of coeliac disease in Sheffield is 1% [24]. Thirty-three patients out of 1,000 had type 1 diabetes mellitus coexistent with coeliac disease (3%). Compared to the background population 12/1,200 (1%), this gives an odds ratio of 3.4 (95% confidence interval: 1.7 to 6.6). Stepwise multiple logistic regression using 13 variables including age, gender, HbA1c, Hb, creatinine and TSH, the only significant variables were positive EMA and positive tTG.
Taken together, this means that GI symptoms are common in type 1 diabetes and exocrine pancreatic symptoms are more common in type 1 diabetes than controls. This is associated with worse HbA1c and quality of life. Also, celiac disease is common in type 1 diabetes. Moreover, it is known regarding celiac and pancreatic insufficiency that celiac disease classically is associated with exocrine pancreatic insufficiency [25]. It has been speculated if this is an autoimmune phenomenon, is due to impaired CCK release [26, 27], to impaired plasma peptide YY release [28] to malnutrition [29], or just is a subclinical pancreatitis.
In a new register study it was found that in a population of 14,239 celiac patients and 69,381 matched controls celiac disease was associated with acute pancreatitis (hazard ratio: 3.3; 95% confidence interval: 2.6 to 4.4) and chronic pancreatitis (hazard ratio: 19.8; 95% confidence interval: 9.2 to 42.8) (the results were adjusted for alcohol and gallstones). From Sheffield it has also been reported [30] that autoimmune pancreatitis may account for 5% of chronic pancreatitis - elevated IgG (notably IgG4) was than the criterion. Celiac disease and exocrine pancreatic insufficiency was found in 11 patients, and celiac disease without exocrine insufficiency in 3.
The author concluded that:
- exocrine pancreatic insufficiency is prevalent in celiac disease (15%);
- about one third of celiac disease patients with continued steatorrhea or diarrhea have exocrine pancreatic insufficiency;
- treatment brings significant improvement in about 90% of cases;
- IgG levels are higher in celiac disease patients with pancreatic insufficiency compared to age and gender matched controls with celiac disease alone.
How Frequent Is Type 3c Diabetes Mellitus?
Philip D Hardt (Giessen, Germany) had given himself the task to discuss how frequent type 3c diabetes really is. He started be reminding the audience that it has now been accepted that exocrine pancreatic insufficiency is very frequent in type 1 (about 50%) and type 2 diabetic patients (about 32%). There are several pathophysiological concepts that may explain the pancreatic insufficiency in diabetes mellitus:
i) insulin has a trophic effect on pancreatic acinar tissue (insulin-acinar portal system) and its lack may cause pancreatic atrophy [31, 32];
ii) islet hormones have regulatory functions on exocrine tissue which may be impaired [33, 34];
iii) diabetic autonomic neuropathy may lead to impaired enteropancreatic reflexes and exocrine dysfunction [35];
iv) diabetic angiopathy may cause local microangiopathy followed by pancreatic fibrosis and atrophy [36, 37];
v) elevated hormone and peptide concentrations (glucagon, pancreatic polypeptide P, somatostatin) may suppress exocrine function [32, 38, 39, 40, 41];
vi) simultaneous damage of exocrine and endocrine tissue:
- viral infections [42, 43, 44];
- simultaneous damage by autoimmunity [45, 46];
- genetic changes affecting exocrine and endocrine tissue [47];
- altered beta cell regeneration from exocrine and ductal tissue;
vii) “it is also possible that some of the diabetics are actually patients with chronic pancreatitis”. It is this that we would emphasize [48].
The fact that pancreatic diseases can cause diabetes mellitus has long been known and therefore pancreatic diabetes has been included into the latest diabetes classification [49]:
1. type 1 diabetes mellitus;
2. type 2 diabetes mellitus;
3. other specific types:
- genetic defects of beta-cell function;
- genetic defects in insulin action;
- diseases of the exocrine pancreas (pancreatitis, trauma/pancreatectomy, neoplasia, cystic fibrosis, hemochromatosis, others);
- endocrinopathies (acromegaly, Cushing's syndrome, glucagonoma, pheochromocytoma, hyperthyroidism, somatostatinoma, aldosteronoma, others);
- drug- or chemical-induced (vacor, pentamidine, nicotinic acid, glucocorticoids, thyroid hormone, diazoxide, beta-adrenergic agonists, thiazides, phenytoin, alfainterferon, others);
- infections (congenital rubella, cytomegalovirus, others);
- uncommon forms of immune-mediated diabetes;
- other genetic syndromes sometimes associated with diabetes (Down’s syndrome, Klinefelter's syndrome, Turner's syndrome, Wolfram syndrome, Friedreich's ataxia, Huntington's chorea, Lawrence- Moon Beidel syndrome, myotonic dystrophy, porphyria, Prader-Willi syndrome, others);
4. gestational diabetes mellitus.
The diabetes prevalence in the U.S. is well calculated for 2007. Under 20 years of age it is 176,500 affected individuals (0.22%), age 20 years or older there are 20.6 millions (9.6%), and age 60 years or older 10.3 millions (20.9%). Type 1 is 5-10%, type 2 is 90-95%, and other types are less than 5%. In the Western literature pancreatic diabetes is believed to account for only 0.5-1.2% of all patients with diabetes mellitus [50, 51], and in Japan 1.69% of 17,500 diabetic patients had pancreatic diabetes [52].
The prevalence of diabetes mellitus in acute pancreatitis is about 50% if defined as impaired glucose tolerance during acute phase whereas persisting diabetes is found in 1-5% [53, 54]. The prevalence of diabetes mellitus in chronic pancreatitis is 40-70%, and in chronic-calcifying pancreatitis up to 90% [55]. It has been questioned how type-3 diabetes mellitus could be frequent if the incidence of chronic pancreatitis is believed to be as low as 0.2 to 8 per 1,000 inhabitants in clinical studies [56, 57]. According to Lankisch et al. [58] the incidence is 6.4/100,000/year, and the prevalence is about 27.4/100,000 (0.027%) [57] or 0.6-8/1,000 (0.06-0.8%) [56]. In contrast to these clinical studies the prevalence of severe exocrine pancreatic insufficiency (elastase less than 100 μg/g in stool) was 10.9% in males aged 55-59 years and 5.1% in the whole group (men and women) of 914 asymptomatic elderly subjects (50-75 years) [59]. Chronic pancreatitis and type 3 diabetes mellitus appear to be underestimated in clinical studies compared to autopsies. In 3,821 autopsy cases 5.3% had signs of chronic pancreatitis in nondiabetic persons at autopsy but in 11.2% of diabetic persons [60]. In another study of 394 autopsy cases 2 cases (0.5%) had clinical chronic pancreatitis but 13% had chronic pancreatitis at autopsy. Nineteen percent of patients with chronic pancreatitis at autopsy had clinical diabetes, whereas 7% of cases without chronic pancreatitis at autopsy had clinical diabetes [61].
This means that if about 10% of the general population show chronic morphologic and functional changes of the pancreas (whether it should be called chronic pancreatitis or not) and if 20% of those developed diabetes mellitus, the prevalence of pancreatic diabetes should be expected to be about 2% of the population. However, the diagnosis of chronic pancreatitis might be missed in clinical practice as symptoms of exocrine disease are not specific in the early stages of chronic pancreatitis. Also, diagnostic procedures have historically been rather invasive (ERCP, direct function tests) and therefore their use has been restricted to obvious indications. Moreover, endocrine dysfunction should be expected to be diagnosed early and might be the first symptom recognized by patients and physicians.
In a reclassification study of diabetics the Giessen Group suggested that about 8% of the patients with diabetes mellitus suffer from type 3c diabetes. However, this study might underestimate the real percentage, since it was done retrospectively and information on the exocrine pancreas was available only in a minority of patients.
Assuming that about 10% of the general population show chronic inflammation of the exocrine pancreas and only 20% of those developed diabetes the prevalence of type 3c diabetes mellitus should be expected to be about 2% of the population. Since diabetes mellitus affects about 7.6% of the population (more than 40 years of age in Germany) pancreatic diabetes should be expected to cause 26.6% of all diabetes cases.
In consequence, type 3c diabetes mellitus appears to be more frequent than type 1 diabetes mellitus and therefore it deserves a little more attention than what it had got to date.
Damage to Exocrine and Endocrine Pancreas by Genes
These issues were covered by Pål Njölstad (Bergen, Norway). There are primary exocrine and primary endocrine pancreatic diseases, but also developmental defects such as IPF-1 mutation. Today several mutations in pancreas progenitor cell genes are known (modifying differentiation to exocrine and endocrine cells), e.g.:
- IPF-1 [62];
- HNF1B [63];
- PTF1A [64];
A lack of IPF1 leads to pancreas agenesis, which is well demonstrated and well accepted. Mutations in HNF1B MODY lead to aplasia only of dorsal pancreas. In a clinical and radiological study (CT, MRCP) in two families including five subjects, elastase measurement showed moderate to severe exocrine dysfunction in all subjects, and aplasia of dorsal pancreas in all five subjects.
The Norwegian research group has especially worked on mutations of carboxyl-ester lipase (CEL), which is a component of pancreatic juice. It hydrolyzes cholesterol esters in the duodenum and is mainly expressed in the exocrine pancreas and in mammary glands. The enzyme is not expressed in the beta-cells. The gene is extremely polymorphic, mainly because of a variable number of tandem repeats (VNTR) in the last exon. As mutations in the gene encoding carboxyl-ester lipase cause dysfunction in endocrine pancreas and early onset diabetes, even though it is expressed in exocrine pancreas, its identifications challenges many of today’s concepts of interactions between exo- and endocrine pancreas.
On the protein level the mutation leads to reduced stability and secretion of the enzyme. The highly polymorphic wild type protein (but not the mutant version) can be detected in urine. Radiological investigations in children with CEL mutations showed regarding ultrasound (performed on the pancreases of 11 non-diabetic, mutationpositive subjects, more than 20-year-old) nonsignificant trend towards smaller size, and significant differences in signal intensity. MRI (VIBE) showed differences in signal intensity suggestive of lipomatosis.
Regarding the cellular models of CEL MODY is approaching the disease mechanism. Nglycosylation in endoplasmatic reticulum is essential for correct folding and secretion. Moreover, O-glycosylation in Golgi masks PEST sequences that are signals for rapid degradation, and phosphorylation in trans- Golgi depends on carboxyl-ester lipase and CEL/Grp94 secreted via condensing vacuoles and zymogen granules. Mutant CEL is aggregating in a subcellular compartment. There are two mouse models available, the CEL knock-out mouse, and mouse made transgenic for the family’s mutation (lox-stoplox/ CRE system). Knockout of CEL in mice seems to promote a mild diabetic phenotype in female mice on chow diet but does not reproduce the phenotype of human CEL MODY. The full CELKO phenotype may require an interaction between this pathway and dietary or other environmental factors for expression of the disease.
There are also well described insulino-acinar effects, meaning that the endocrine pancreatic hormones influence exocrine pancreas. Example of this is that insulin and PP induce growth of exocrine pancreas whereas glucagon and somatostatin do not [10, 31, 67].
Exocrine dysfunction has also been evaluated in HNF1A MODY patients recruited from the Norwegian MODY Registry: 63 HNF1A MODY patients, 140 patients with type 1 diabetes, and 78 controls. It was found fecal elastase deficiency in 13% HNF1A MODY patients versus 4% in controls [68]. With regard to pancreatic exocrine disease in diabetes there are several good studies published (Table 1).

However, it is not only endocrine cells that influence the exocrine cells, but also the reverse. Examples of exocrine diseases that influence the endocrine function are:
i) monogenic diseases:
- cystic fibrosis;
- Schwachman-Diamond syndrome;
- Johanson-Blizzard syndrome;
- CEL MODY;
ii) chronic pancreatitis;
iii) hereditary pancreatitis P;
iv) pancreatic cancer;
v) pancreatectomy.
Regarding the monogenic diseases it is known that the mutated gene CFTR in cystic fibrosis leads to 60% impairment of the exocrine function [70] and 76% of the subjects are diabetics by the age of 30 [71], whereas in the Schwachman-Diamond syndrome with the SBDS gene mutation exocrine pancreatic function is low since birth and there are case reports on survivors with diabetes [70]. In Johanson-Blizzard syndrome the URB1 gene is mutated and exocrine pancreatic function is low since birth and there are case reports on survivors with diabetes [70] and in CEL MODY all exocrine pancreatic function is lost by year 20, and all are diabetics by the age of 50 [47].
Impairment of Beta Cell Homeostasis and Type 3 Diabetes
Mathias D Brendel (Giessen, Germany) discussed transdifferentiation and transduction of cell lines. It is well described that exocrine pancreatic cells may transdifferentiate to hepatic phenotypes and hepatic to pancreatic phenotypes. However, endocrine transdifferentiation of pancreatic precursor cells both in vivo and in vitro has also been observed [72, 73, 74, 75, 76].
Tag Ngn3 expressing cells:
- ngn3+ cells originate in hormone negative cells near ducts and become islets;
- duct ligation activates ngn3 expression and increases beta cell mass;
- new islets after duct ligation derive from hormone-secreting progenitors in ducts;
- knock down of ngn3 impairs duct ligationinduced beta cell generation;
- ngn3+ cells isolated from adult pancreas have embryonic islet progenitor phenotype;
- ngn3+ sorted cells (adult pancreas) differentiate into functional islets in vitro.
Regarding transduction of pancreatic and intestinal precursor cells into insulinproducing cells, it has been shown that overexpression of PDX-1 in ductal cell line leads to GLP-1 induced insulin secretion [77], whereas hepatic overexpression of PDX-1 in Xenopus leads to differentiation into exocrine and endocrine pancreatic cells [78]. Overexpression of PDX-1 in intestinal crypt cells leads to insulin secretion [79]. Transduction of gut K-cells lead to insulin secretion [80].
Associations between ducts and endocrine pancreas are not exceptions, but the rule. Single insulin-producing cells are seen within the ductal lining in rats in about 1% of the cells [81] and in humans in about 15% [82]. In the rat the incidence of PP- and somatostatin-cells increase towards distal ducts and common bile ducts, where an “open type“ of cells containing secretory granules with surface facing the ductal lumen.
Brendel concluded that:
- the pancreas anlage is derived from common pluripotent stem cell tissue;
- regeneration of pancreas tissue can use common progenitors
pancreatic “compartment“ tissue can transdifferentiate and be transduced into each other (i.e. pancreas plasticity);
disturbed regeneration can affect both endocrine and exocrine pancreas;
- close anatomical and physiological interactions between endocrine, ductal and acinar tissue are established, but more may be there to uncover;
- endocrine-exocrine-ductal axis is required for optimal pancreatic function.
Peculiarities in The treatment of Type 3 Diabetes Mellitus
Nils Ewald (Giessen, Germany) introduced the first case report of a patient with diabetes mellitus due to chronic pancreatitis. It is probably the report of Cawley from 1788 about “a 34-year-old man accustomed to free living and strong corporeal exertions in the pursuit of country amusements…“ [83].
Regarding the pathophysiology in type 3 diabetes there are some peculiarities:
i) decreased or absent insulin secretory capacity [84];
ii) decreased or absent glucagon secretion:
- insulin-induced hypoglycemia and insulin withdrawal did not stimulate glucagon secretion in the secondary diabetic patients in contrast to comparable type 1 diabetics, [85];
- blood glucose counter regulation is intact in the secondary diabetics due to preserved (yet blunted) catecholamine secretion [85, 86];
iii) increased plasma concentration of somatostatin [87]:
- may contribute to a reduction in overall blood glucose level in patients without endogenous insulin secretion due to inhibition of glucagon secretion
iv) diminished incretin release due to exocrine pancreatic insufficiency [88];
v) decreased PP secretion [89];
vi) PP secretion absent in chronic pancreatitis without endogenous insulin production;
vii) PP cells seem to be as vulnerable as the beta-cells to the destructive processes characterizing chronic pancreatitis, whereas alpha-cells preserve secretory capacity to a greater extent than PP-cells and beta-cells;
viii) loss of hepatic insulin receptor expression and availability and impairment in hepatic IR function (phosphorylation and endocytosis) [89];
ix) diminished hepatic IR expression in chronic pancreatitis appears to be because of PP deficiency;
x) trouble with concomitant alcohol abuse (chronic pancreatitis patients):
- hepatic disease;
- effects of alcohol;
- non adequate food intake and poor nutrition. Taken together pancreatic diabetes has been believed to be a brittle diabetes in the past. Several special suggestions for the treatment of type 3 diabetes have been made:
- “the characteristics of pancreatic diabetes suggest that a conservative approach be taken in regard to intensive insulin therapy and tight blood glucose control” [90];
- no metformin should be used in patients with alcohol abuse (cave: lactate acidosis);
- alpha-glycosidase inhibitors might aggravate existing GI-complaints (due to exocrine insufficiency);
- sulfonylureas are not indicated as the betacells are destroyed anyway, and they increase the risk of hypoglycemia [91];
- a relevant high amount of patients is generally treated with diet and oral antidiabetic drugs [92, 93].
However, controlled clinical trails about the effects and risks of treatment with oral antidiabetic agents or insulin treatment in type 3 diabetes mellitus are nearly missing.
Recently, some new treatment options were proposed from the Queen Elizabeth Hospital, Birmingham, [94] based on a retrospective analysis of all patients undergoing total pancreatectomy (1989-2003) with a comparison made against a matched type 1 diabetic population. The study included 47 patients with a median follow-up of 50 months. There was no significant difference between median HbA1c of the study group (8.2%) and the control (8.1%). The majority of patients reported diabetic control and daily performance as excellent or good. Resection for pancreatitis gave significantly poorer subjective control than those resected for malignancy. The authors’ conclusion was that “diabetes after total pancreatectomy is not necessarily associated with poor glycemic control and in the majority results in equivalent biochemical control compared to a normal type 1 diabetic population.”
In a German prospective study of 35 patients with diabetes type 3 followed-up for 18 months (Nauck et al., unpublished data) a comparison of matched type 1 and type 2 diabetic patients was made. It was found that insulin treated type 3 diabetes mellitus is “not” characterized by a higher risk of symptomatic or severe episodes of hypoglycemia as commonly (“theoretically“) supposed.
Regarding pancreatic enzyme therapy in patients with diabetes mellitus and exocrine pancreatic insufficiency there are some studies currently available. In one there was no positive effect on HbA1c, but less stable control in patients with diabetes in chronic pancreatitis when enzymes were added or removed [95]. On the other hand there were positive effects on HbA1c and more stable disease in patients with diabetes mellitus in tropical pancreatitis [96], while no positive effect on HbA1c but more stable control were reported in patients classified as insulinopenic diabetes in chronic pancreatitis [97].
In a German multicenter study with 80 insulin-treated patients, it was shown that pancreatic enzyme replacement therapy in insulin-dependent diabetes mellitus does neither prove to have positive nor negative effects on glucose control. However, it may be a positive effect on GI-symptoms and steatorrhea, and positive effect on qualitative malnutrition, e.g. alterations of vitamin D3 metabolism in young women with various grades of chronic pancreatitis [98]. Whether there are special positive effects in patients with residual beta-cell function is still not known.
Incretins in Type 2 and Type 3 Diabetes Mellitus
Filip K Knop (Gentofte, Denmark) discussed the role of incretin hormones. Preceding absorption carbohydrates are cleaved to glucose in the gastro-intestinal tract. Than, blood sugar concentration rises and pancreatic insulin secretion is started. Than, insulin facilitates transportation of glucose to intracellular compartment and blood sugar concentration normalizes again. The “incretin effect” is the difference in insulin concentration after an oral versus an intravenous glucose load (lower insulin response after an intravenous load, but the same effect on the blood sugar levels). In healthy subjects the incretin effect is about 70% of the response. Two incretin hormones are known; glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These hormones are secreted from endocrine cells in the small intestinal mucosa (GIP: K-cells; GLP-1: Lcells). The secretion stimulus are intraluminal nutritional components. Both hormones are degraded rapidly by dipeptidyl peptidase 4 (DPP-4) after 7 and 1.5 minutes, respectively [99].
GLP-1 reduces blood sugar by pushing glucose-dependent insulin secretion, increasing the somatostatin secretion (that lowers the glucagon secretion that decreases gluconeogenesis in the liver). GLP-1 also decreases gastric emptying and acid secretion. On the beta-cell GLP-1 increases glucose sensitivity, insulin secretion and the insulin synthesis (transcription of the insulin gene). This leads to an increased mass of beta-cells with improved function. The effects of GLP-1 and GIP are shown in Table 2.
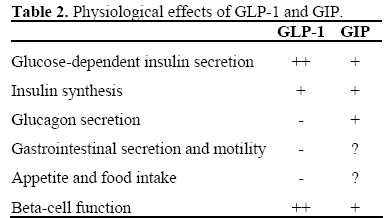
In diabetes type 2 there is a reduced incretin effect (70 to 30%) and a decreased insulinotropic effect of GIP. It is not known whether this is the cause or the consequence of the diabetic state. This has been studied in three studies:
- there are increased postprandial responses of GLP-1 and GIP in patients with chronic pancreatitis and steatorrhea following pancreatic enzyme substitution [100];
- there is a reduced incretin effect in type 2 diabetes (cause or consequence of the diabetic state?) [101];
- the insulinotropic effect of GIP is impaired in patients with chronic pancreatitis and secondary diabetes mellitus as compared to patients with chronic pancreatitis and normal glucose tolerance. [102].
In some patients with chronic pancreatitis and exocrine pancreatic insufficiency a normal postprandial secretion of GLP-1 and GIP was shown. However, enzyme supplementation increased the secretion of the incretin hormones GLP-1 and GIP resulting in increased postprandial insulin secretion. While patients with chronic pancreatitis and normal glucose tolerance exhibited an intact incretin effect, patients with chronic pancreatitis and secondary diabetes exhibited decreased incretin effects (to the level of patients with type 2 diabetes). Reduced incretin effects are not a unique type 2 diabetes phenomenon, but rather a consequence of the diabetic state. Thus, the reduced secretion of GLP-1 and/or GIP seems not to explain the reduced incretin effect in patients with diabetes.
Incretins: from Concept to Treatment
Baptist Gallwitz (Tübingen, Germany) discussed possibilities to utilize GLP-1 action therapeutically. There are two obvious solutions: long acting DPP-4 resistant GLP-1 analogs or incretin mimetics and DPP-4 inhibitors as incretin enhancers [103, 104]. DPP-4 is a serine-protease of the prolyloligopeptidase enzyme family and exists in 2 forms: membrane bound (endothelium) and soluble (plasma) [105, 106, 107]. There are several DPP-4 inhibitors available, e.g. vildagliptin (Galvus®, Novartis), sitagliptin (Januvia®, MSD), alogliptin (Takeda), and saxagliptin (AstraZeneca/Bristol Myers Squibb) [108]. A summary of clinical studies with DPP-4 inhibitors show:
- lowering of fasting- and post-prandial glucose in monotherapy;
- lowering of HbA1c comparable to metformin in monotherapy;
- lowering of HbA1c in combination with metformin;
- lowering of HbA1c in combination with pioglitazone;
- lowering of HbA1c comparable to glipizide as add on to metformin;
- good tolerability and safety;
- hypoglycemia incidence comparable to placebo or metformin;
- weight neutral.
There are certain differences with different approaches to incretin therapy for diabetes (Table 3).

Gallwitz explained that although incretin therapy is already here, there are still more questions to be answered, such as the different mode of action of incretin based therapies in clinical use. The possible role of incretin based therapies in prediabetes or early stages of diabetes is still unclear, as is the influence of incretin based therapies on disease progression. There are discussions on other possible indications for incretin based therapies (e.g., transplantation, cardioprotection) and the differences among various DPP-4 inhibitors. We will probably hear more on this in the nearest future.
How Frequent is Chronic Inflammation of the Pancreas?
J Enrique Dominguez-Munoz (Santiago de Compostela, Spain) was given the question on how frequent chronic inflammation of the pancreas is and how a chronic inflammation should be defined. There are obviously several accepted (or possible) etiologies of chronic inflammation of pancreas, such as alcohol and tobacco, recurrent acute pancreatitis, genetic mutations and immunological alteration, hypercalcemia, chronic renal failure, and ductal obstruction. Despite this, it is not known if chronic inflammation persists from very early to very advanced disease. In one study [109] it was shown that in cases with inflammatory infiltrates at EUS-guided biopsy there was fibrosis in only 64%, and affected ductal epithelium in 64 and pancreatic acini in 36%, respectively. It may also be underlined that the incidence figures from different countries in Europe differ significantly, from 1 new case of chronic pancreatitis per 100,000 inhabitants (England) to 13 (Spain), and 23 (Finland). The prevalence of chronic pancreatitis has been calculated to be 15.8 per 100,000 inhabitants in France and 18.3 in Spain. It is significantly higher in Japan.
For all these prevalence and incidence figures it is, however, essential to define the onset of the chronic pancreatitis or the inflammation. Especially, the target population for investigations must be carefully defined (alcoholics, “normals”, persons with defined grade and duration of abdominal pain of defined quality, etc.). It must also be declared if chronic pancreatitis is equivalent with low fecal elastase, calcifications on X-ray or something else. It is also well known today that age and smoking are independent risk factors whereas ACE inhibitor intake seems to be a protective factor, while alcohol consumption might not be as important as previously believed [59]. In alcoholics the frequency of chronic inflammation in the pancreas ranges from 3.5 to 72%. The frequent finding of exocrine insufficiency in the elderly implies that chronic inflammation might not be a “disease” but a normal feature of the human pancreas (at least at higher ages). Early chronic pancreatitis is rarely suspected when pain is mild or absent and when symptoms are unspecific (“dyspepsia”) in the absence of steatorrhea. In patients classified as “functional dyspepsia” Smith et al. reported an abnormal Lundh test in 27% [110]. Furthermore, the differential diagnosis between acute pancreatitis and acute attack of chronic pancreatitis is difficult, but alcohol intake may serve as a “red light” (but only in some cases).
Early chronic pancreatitis remains a diagnostic challenge as there is no gold standard for the diagnosis and pancreatic biopsy is risky and impractical. Moreover, biopsy is reliable only in positive cases as chronic pancreatitis frequently has a patchy distribution. There is a mainly unknown feature-to-feature correlation of imaging procedures and histopathology, with good correlation between morphological and histological findings only in normal pancreas and severe chronic pancreatitis. However, today endoscopic ultrasonography allows accurate imaging of both pancreatic parenchyma and ducts, but it has a limitation in its interindividual variability. An evolution of EUS is endoscopic elastography. Elastography today is the best available measures to differentiate early chronic pancreatitis from normal pancreas [111]. Using EUS as a diagnostic standard Lee described that among 200 patients with uninvestigated dyspepsia he observed 3% with definite signs of chronic pancreatitis [112].
The take-home-messages from Dominguez- Munoz were:
i) reported data on incidence and prevalence of chronic pancreatitis are unreliable and highly variable:
- advanced disease;
- alcohol as “red light”;
ii) early diagnosis of chronic pancreatitis is a challenge:
- chronic pancreatitis is clearly underdiagnosed;
iii) EUS and EUS-elastography may open a window for the future.
Is Pancreas Fibrosis as Frequently Observed in the Elderly a Disease yo Be Treated?
Matthias Löhr (Stockholm, Sweden) first attempted to find out the incidence of pancreatic fibrosis, but found no reliable data. The nearest to be found was figures for newly diagnosed chronic pancreatitis: 4 per 100,000 inhabitants, but less than 1 per 100,000 in individuals older than 59 years of age.
The age distribution may help to sort out some problems (and delineate a few more). In a large material of chronic pancreatitis [113] those with alcoholic origin (84% of all) had a mean age of about 47 years, whereas hereditary pancreatitis and cystic fibrosis (2% of all) had a median age of 25-29 years, and the idiopathic patients (9% of all) had one peak at 50, and another one at 70, years. In another material [114] it was made a difference between chronic pancreatitis with early onset and those with late onset (Table 4).

Of certain interest is that the survival curves look the same with the exception of the 15- year shift. The author also discussed if less calcification is equivalent to more fibrosis, but that is still not settled.
The extracellular matrix has been extensively studied in chronic pancreatitis. There is little extracellular matrix (ECM) in pancreas. In chronic pancreatitis the pancreatic duct cells produce hyaluronic acid, which serves as a reservoir for FGF. Also, the levels of growth factors (TGF-beta, PDGF, and FGF), produced by monocytes and macrophages, are elevated in the stroma, which is mostly produced by the pancreatic stellate cells (PSC) [115, 116, 117, 118].
By proteomics and transcriptomics it is verified that some genes are up- or downregulated in chronic pancreatitis [119, 120]. Among the upregulated are:
- cathepsin B;
- interleukin receptor 2, 7, and 10 alpha;
- MHC class II DR alpha, beta1, beta3, and beta5;
- TGF beta1;
- TNF receptor (1B, 7, and 17);
- collagen type III;
- lactoferrin;
- granozyme/serine protease;
- IgG.
Downregulated are:
- albumin;
- carbonic anhydrase II;
- trypsinogens;
- elastase;
- PSP;
- Reg 1A and B;
- amylase, lipase;
- SPINK1.
Today the sequential pathogenesis of pancreatic fibrosis can be described as: normal pancreas; then, influence of noxious stimuli like alcohol and smoking; then, initial damage of acini and duct cells; then, invasion of inflammatory cells; then, cytokine release (TGFbeta1); then, proliferation of the matrix cells by pancreatic stellate cells; then, desmoplacia. In long-term alcohol drinking rats there are some visible differences, but there is no fibrosis. Possibly the difference with regard to the human situation is that smoking is missing. When pancreas is exposed to alcohol it has been found 61 upregulated genes and 42 downregulated genes. Those that are upregulated at least 3- fold are CSPG-6, collagen type 1, alpha 1, prolin-rich protein, Reg1, and Reg3a.
It is well described that there are pancreatic intraductal neoplasias (PanIN) present in chronic pancreatitis [121, 122]. TGF-beta1 expression is present already in PanIN 1B. It may also be noted that K-ras positivity is dependent on the grade of PanIN in pancreatic cancer, but not in chronic pancreatitis. In chronic pancreatitis, K-ras positivity is dependent on the duration of disease [123]. Most smokers have grade III-IV PanINs in chronic pancreatitis, and most grade IV in chronic pancreatitis are K-ras positive.
There is correlation between K-ras and p53 in chronic pancreatitis with the severity of chronic pancreatitis (Table 5) [124].

There is a correlation between pancreatic age and volume, with a gradual decrease in volume from age of 40 years [125]. There is also a slight but significant decrease in lipase concentration in lipase concentration and lipase output [126]. According to Rothebacher et al. in an investigation of 914 individuals insufficiency is also more common with age (Table 6) [59].

Insulin is trophic for the exocrine pancreas [7]. On the other hand, pancreatic atrophy in longstanding insulin-dependent diabetes mellitus has also been found [125, 126]. This is of certain interest even though there is an acinar atrophy, but little fibrosis (i.e. this is not chronic pancreatitis). In these cases microangiopathy and a reduced blood flow are found.
In elderly persons, the pancreas may contain ducts narrowed by ductal papillary hyperplasia, a lesion that has now been termed pancreatic intraepithelial neoplasia type 1B. In association with this lesion, there may be patchy lobular fibrosis in the periphery of the pancreas. The fibrosis affects the lobes that are drained by ducts showing PanIN-1B lesions. The extent of this lobular fibrosis varies from person to person; the most severe form and the highest incidence (up to 50%) are found in persons older than 60 years [127]. The pancreatic ductal hyperplasia and fibrosis is associated with age (Table 7) [128].

PanIN is thus more frequent in the elderly. The fibrosis may be diagnosed by endosonography, which is capable to detect early changes both in the parenchyma and in the ducts with a sensitivity and specificity both greater than 95%. However, systematic prospective studies with sequential investigations are missing. There are indications that EUS elastography is even better [129].
The extracellular matrix in chronic pancreatitis shows increased P-III-P, laminin, and hyaluronic acid, but only hyaluronic acid can differentiate between a bout of chronic pancreatitis and resting chronic pancreatitis, and might be a marker of early chronic pancreatitis. Regarding this there is no relation to age [130].
At present there is no antifibrotic medication available. Instead, if identifiable, underlying disease to the pancreatitis must be treated, such as alcoholism, gallstones, autoimmune disease etc. Finally Löhr emphasized the M-ANNHEIM classification of chronic pancreatitis (a classification taking into account etiology, clinical course, and severity) [131]:
M Multiple risk factors such as:
A Alcohol;
N Nicotine;
N Nutritional and metabolic factors (e.g. hyperlipidemia);
H Hereditary factors (e.g. SPINK1, CFTR, PRSS1);
E Efferent duct system (e.g. pancreas divisum);
I Immunological factors (e.g. autoimmune pancreatitis);
M Miscellaneous rare factors (e.g. hypercalcemia).
ACE and Inflammation of the Pancreas
A new approach to the problem was brought up by Britta Fischer (Giessen, Germany): the influence of the (local) renin-angiotensinsystem on the pancreas, which might be one oft he most important contributions to the conference. Since many years it has been well known that the renin-angiotensin-system plays an important role in the pathophysiology of the cardiovascular system, especially as the most important regulator of blood pressure and fluid balance. Angiotensinogen is synthesized in the liver. Renin from the kidney will turn it into angiotensin I and the angiotensin-converting enzyme (ACE) in the lungs to angiotensin II, which acts on AT-1 receptors on the endothelium.
Local renin-angiotensin-systems are found in many tissues (heart, adipose tissue, pancreas, etc.) but with somewhat different functions: regulation of cell growth, differentiation, proliferation, regulation of ROS-generation, upregulation in inflammation and so on. Its effects in the pancreas had now been investigated.
The renin-angiotensin system is upregulated by different conditions such as hypoxia, pancreatitis, pancreatic cancer, diabetes mellitus and after islet transplantation. Angiotensin II dose-dependently stimulates the release of digestive enzymes from acinar cells probably via calcium [132] and regulates islet glucose-stimulated insulin secretion (shown in mice and rats) [133, 134]. ACE is found evenly distributed at the vessels of the exocrine and endocrine tissue. In humans there was little variation in the staining intensities between the 20 pancreata studied. In some pancreata, there was an intensive selective staining of all islets in the pancreas. In other pancreata non-inflamed islets were unstained, whereas cells in inflamed islets could be stained. AT-receptor 1 is upregulated in islet culture and co-localizes with glucagon.
There might be some therapeutic options with RAS-inhibition in chronic pancreatitis and diabetes. ACE-inhibitor intake might be a protective factor for developing pancreatic insufficiency [59] and it improves pancreatitis-induced injury probably through inhibition reactive oxygen species in rats [132, 135]. As it revents stellate cells activation it is antifibrotic in the rat [136, 137]. It seems as if low ACE activity protects the beta cell from decline in function in adult type 1 diabetes mellitus. Hyperglycemia and hyperinsulinemia enhance pancreatic stellate cell activation of the renin-angiotensinsystem, and this leads to islet restricted fibrosis [138]. In clinical studies pharmacological blockade of these pathways reduces the incidence of type 2 diabetes in high risk patients with cardiovascular disease [139]. Islet cell fibrosis can be attenuated by ACE-inhibition in the rat model [138].
As conclusion, Fischer advocated that:
- there is a functional renin-angiotensinsystem in endocrine and exocrine pancreas with a high individual variability in humans;
- the expression of the components are different among species;
- blockade of this system attenuates chronic pancreatitis and reduces incidence of type 2 diabetes mellitus.
Genomics and the Metabolic Hypothesis of Chronic Pancreatitis
Fransesco Marotta (Milan, Italy) described an Italian study on chronic pancreatitis started in 22 centers in 2001 with regard to epidemiological, clinical, diagnostic and therapeutic aspects. The study included 1,068 patients by the end of 2006. The mean age of the included patients was 52 years and 72% were men. The age of onset of the disease was 45 for men and 42 for women. Only 61% of the diseased drank alcohol and of all only 40% drank at least 80 g alcohol per day. Eighty percent of the men smoked, but only 45% of the women. Regarding pathophysiology 1.5% of the patients had autoimmune pancreatitis, and 1.2% had mutations of CFTR. Twenty-seven percent of the patients had diabetes, but of the patients with autoimmune pancreatitis only 12% had diabetes. On the other hand 59% had had cholecystectomy and 18% had biliary stones. Twelve percent of these patients had calcifications and 29% had steatorhea. Marotta also made an interesting cartoon on the “omics” of nutrition (Table 8).

It is well known that there are several connections between obesity, insulin resistance, metabolic syndrome and diabetes. There are many proteins known to take part in this connections, such as PPARg2, leptin, leptin receptor, MCR4, POMC, UCP1, beta-2 adrenergic receptor, alpha-2 adrenergic receptor, CETP, Apo A, B and E, GNBP, LPL, ACE, eNOS, interleukin-1 and interleukin-6, TNF-alpha, Vit DR, resistin and USF1.
Regarding chronic pancreatitis and diabetes it is known that patients with diabetes mellitus associated to chronic pancreatitis have an accelerated atherogenesis even though they have lower LDL cholesterol [140]. Low level of linoleic acid is associated to higher incidence of CAD [141] and a higher risk to develop an insulin-resistance syndrome [142]. The Milan group tried therapeutic effects of red palm oil, the composition of which is shown in Table 9.

In the red palm oil there is much more tocopherols, tocotrienols, and carotenoids compared to other dietary oils. In patients with chronic pancreatitis effects of red palm oils could be shown in many ways:
- the index of EFA deficiency 16:1(n7)/18:2(n6) was the same as in the controls, whereas in the untreated patients with chronic pancreatitis the index was significantly higher;
- alpha-tocopherol concentrations in chronic patients was as high as in controls, whereas in the untreated patients with chronic pancreatitis it was significantly higher.
TNF-alpha, fractalkine (sFKN), and interleukin-6 showed the same pattern. There are indications that fractalkine may be a marker of progressing early-stage chronic pancreatitis [143] possibly working through the pancreatic stellate cells. A nutritional approach with phytotherapeutic agent K- 17.22 was also discussed as it delays the onset of experimental spontaneous chronic pancreatitis. A strictly-controlled herbal remedy (K-17.22, Kyotsu Inc., Tokyo, Japan) has been shown to significantly reduce cytolytic liver parameters in HCV patients [144] and NASH biochemical and histological score [145]. On experimental basis, K-17.22 can significantly reduce CCL4 histological damage with GSH-sparing effect [146].
Marotta ended by some hopes for the future:
- genomic analysis may help identifying patients at higher risk of metabolic syndrome, insulin resistance, obesity, etc.;
- such evaluation may be worthwhile for a better (revisited) etiological sub-grouping of chronic pancreatitis;
- this may lead to an earlier intervention strategy to slow down or halt the onset or progression of the disease.
Aspects of Nutrition in Pancreatic Disease
Elena Rangelova (Stockholm, Sweden) discussed the influence of nutrition on pancreas in health and disease which in turn might influence the interaction between endocrine and exocrine pancreas. She started by showing methods for nutritional assessment:
- anthropometry (BMI, weight loss, skinfold thickness, arm circumference, etc.);
- biochemical markers (albumin, transferrin, RBP, pre-albumin, etc.);
- immune-response tests (delayed hypersensitivity test, lymphocyte counts, etc.);
- body composition tests (whole-body conductance and impedance, neutron activation, etc.);
- functional tests (handgrip dynamometry, adductor pollicis muscle response, etc.);
- nutritional indices (nutritional risk index, prognostic nutritional index, etc.);
- clinical nutritional assessments tools (subjective global assessment: SGA; mini nutritional assessment: MNA; nutritional risk screening: NRS 2000; etc);
- dietary methods.
Using some of these methods the incidence of malnutrition in chronic pancreatitis has been calculated:
- 52% (BMI <20 kg/m2, stool fat excretion rates >10 g/day) [95];
- 61% (BMI <20 kg/m2, weight loss >10% per 3 months) [147];
- 31% and 47% (BMI and albumin, respectively) [148];
- 46% and 37% (SGA: category C and B, respectively) [149];
- 67% (body weight, RBP, prealbumin) [150].
Regarding malnutrition and morbidity in chronic pancreatitis there are no studies on impact of malnutrition on “outcome” of the patients. Moreover, there are no data comparing morbidity in chronic pancreatitis and other patients and healthy individuals for certain pathology. Even though there are no studies on impact of malnutrition on outcome it has been found more frequent cardiovascular lesions and lesions at an earlier age [151]. Also, non-diabetic retinal lesions and functional abnormalities without correlation to vitamin A, zinc or glucose tolerance [152].
Regarding nutrition and digestion in chronic pancreatitis it is necessary to pay attention to the following aspects:
- adequate oral intake and a balanced diet;
- optimal digestion and absorption;
- right GI motility;
- adequate metabolism.
All these correspond to the metabolic demands. Increased metabolic needs increase the energy expenditure. However, in chronic pancreatitis there is always a risk of nutrient insufficiency due to insufficient oral intake, exocrine pancreatic insufficiency (weight loss and nutrient deficiencies), dysmotility, disturbed metabolism (diabetes), and postsurgical problems. At indirect calorimetry it has been found an increased resting energy expenditure in 33% of patients with noncomplicated chronic pancreatitis [153], and 33% hypermetabolic [147]. Resting energy expenditure was significantly higher in malnourished patients with chronic pancreatitis versus normal-weight chronic pancreatitis patients and malnourished controls.
Regarding the nutritional insufficiency it has been shown concomitant with clinical signs there will be a spontaneously modify diet with decreased caloric intake, fat, and alcohol [154]. There is also a Japanese study showing decreased intake of calories and carbohydrates [155], but also increased calories and carbohydrate but mostly in surgical treated patients [156]. In another study a BMI of 20.7±2.6 in chronic pancreatitis patients versus 22.7±3.4 in hospital controls has been shown. This problem is worsened by the pancreatic insufficiency, leading severe undernutrition with primary pancreatic acinar cell dysfunction and decreased secretion of amylase, lipase, and trypsin [157] and lowered bicarbonate, trypsin, and lipase secretion leading to steatorrhea in all [158].
Due to the exocrine insufficiency there can be a lowered cholesterol, HDL-C, Apo-A1, Lp(a) [159]. There is also a deficiency of vitamins and micronutrients, such as vitamins A, D, E, K, B1, B12, folic acid and calcium, magnesium, zinc, and selenium [160]. Especially, there seems to be a deficiency of antioxidants as the intake is decreased regarding vitamin E, riboflavin, choline, magnesium, copper, manganese, and sulfur [161]. In another study the plasma concentrations were impaired concerning selenium, vitamin A and E, beta-carotene, xanthine, beta-cryptoxanthine, and lycopene [162].
In chronic pancreatitis there is an accelerated gastric emptying, but enzyme supplementation normalize gastric emptying [163, 164]. Gastroparesis has been found in small duct chronic pancreatitis in 44% [165], also called gastroparesis diabeticorum. In exocrine disease there is also a delayed gallbladder contraction, that improves with enzyme supplementation [166] and an accelerated small intestinal transit (note: the ileal brake slows down gastric emptying and intestinal transit, for conversion to interdigestive pattern) [167, 168].
The nutritional support should include dietary modifications. It is then known that (for good and bad) stimulation of pancreatic secretion can be modified:
- meals of 500 kcal induce maximal enzyme response [169];
- solid meals induce more sustained enzyme response [170];
- lipids are the strongest stimulants of pancreatic secretion [171];
- long chain fatty acids in the duodenum are more potent stimulants than medium chain fatty acids [172, 173];
- dietary fibers inhibit pancreatic lipase [174];
- jejunal perfusion.
Based on this and the hypothesis that decreased pancreatic stimulation should avoid symptoms such as pain and steatorrhea the common dietary recommendations are:
- frequent low-volume meals;
- low-fat diet (50 g fat per day or 0.5 g per kg BW per day);
- high-calorie (35 kcal/kg/24 h), high-protein (1.0 to 1.5 g/kg/24 h) diet;
- medium-chain triglycerides recommended;
- diet low in fiber;
- alcohol abstinence;
- supplementation of fat-soluble vitamins.
Regarding dietary modifications it should be noted that decreasing the fat content in a diet of a patient who is already losing lipids (steatorrhea) would worsen nutrient and vitamin deficiency [156]. Medium-chain triglycerides are unpalatable as they induce abdominal cramps and diarrhea [175]. The survival of enzyme activities is enhanced by the presence of the corresponding luminal nutrients [176, 177].
It is difficult to stimulate pancreatic secretion in chronic pancreatitis (there is very low exocrine response to endogenous and exogenous stimulation in chronic pancreatitis) [178]. Also, regarding cholecystokinin there is a much lower response to meals (levels increase after enzyme therapy) [179, 180]. The levels decrease with progression of the exocrine insufficiency [173], but application of protease inhibitor camostat did not influence CCK levels [181]. It is unknown if the stimulation of secretion leads to deterioration of disease. The inhibition of secretion with regard to pain has not shown impressive results. Enzyme substitution therapy has not been shown to induce pain relief in most studies [182]. Studies of the somatostatin analogue octreotide also failed to significantly reduce pain syndrome [183] and there has not been found any relationship between pain score and pancreatic pressure [184].
There are no good studies comparing the effects of different dietary regiments on nutritional status, symptoms and outcome. Data on the utility of enteral nutrition in chronic pancreatitis are very insufficient, mostly only small uncontrolled retrospective series. Four studies have been published regarding polymeric formulas (Table 10).

Peptamen plus low-fat diet reduced pain scores by 70% after 10 weeks of therapy [188]. Also, long-term jejunal feeding (after 6 months) gave a pain decrease from 96 to 23%, and intake of pain medication decreased from 91 to 21%, and gastrointestinal symptoms decreased from 90 to 15% [149]. There is almost no data on TPN in chronic pancreatitis.
Rangelova concluded that:
- the incidence of malnutrition in patients with chronic pancreatitis is high, but its impact on morbidity and mortality has not been investigated;
- chronic pancreatitis affects the digestive processes on all levels;
- patients with chronic pancreatitis have latent nutrient and micronutrients deficiencies with unknown consequences even if they seem to respond well to the standard dietary and medical interventions;
- no firm evidence exists for the commonly recommended dietary modifications;
- insufficient data for recommendation of enteral nutritional support and almost lacking for parenteral nutritional support;
- correction of the altered nutritional status may be achieved by close monitoring and adjustment of the enzyme substitution therapy;
- some evidence that improvement of nutritional status may improve the symptoms of disease;
- the difficulties with providing adequate nutritional support and maintaining good nutritional support will be further challenged with the progression of the disease due to the exocrine and endocrine interactions;
- all these nutritional challenges influence also the endocrine steady-state.
Pancreatic Stellate Cells and Pancreatitis
According to David Fine (Southampton, UK) the first paper on pancreatic stellate cells was published in 1989 and in 2006 and 2007 there were more than 40 new reports. There are, however, several questions that are still unanswered, such as: “Where are the “quiescent” pancreatic stellate cells and what are they doing?”; “Are pancreatic stellate cells the source of pancreatic fibrosis?”; and “Does the endocrine environment affect pancreatic stellate cell behavior?”. However, it is known that the quiescent stellate cells may be found around acini and between lobules, but it is a problem that their markers regularly (so far) are destroyed by fixation and frozen sections give poor morphology There is a good recent review on the issue [189].
Maybe the quiescent pancreatic stellate cells are there to maintain tissue integrity, as they store retinoids, retinoid doping favors quiescence in culture and retinoids maintain epithelial integrity and differentiation. There have also been speculations that the stellate cells have neurological functions, and may participate in mediating secretion. We know that there are no acetylcholine receptors present on acinar human cell. But some novel receptors on pancreatic stellate cells have recently been described by Apte et al.. Secretary vesicles can be seen in these cells. It may be questioned if the stellate cells have motor function as they have actin filaments. It is also well described that the cells “cousins”, the hepatic stellate cells, probable regulate hepatic blood flow.
There is circumstantial evidence supporting that there they the source of pancreatic fibrosis:
i) cells in fibrotic bands and within cancers are myofibroblastic;
ii) activated PSC are myofibroblastic:
- isolated by centrifugation;
- isolated by outgrowth;
iii) pancreatic fibroblasts are distinct, and have a different morphology.
However, there are also other possible origins, such as transdifferentiation of other cell types and stem cells (local or hematogenous). There are indications that transdifferentiation may be of importance [190]. In this model there is found hypertension and insulin resistance.
It is interesting that the pancreatic stellate cells exist in a high-insulin environment. Insulin is trophic to activated stellate cells. This may be via classical insulin receptor, IGF-1 receptor, or both. It has been shown that insulin and IGF inhibit pancreatic stellate cell apoptosis.
So, the conclusions of the authors were:
- there is still great ignorance about physiology and pathophysiology of pancreatic stellate cells;
the “quiescent” phase may be important for normal pancreatic structure and function, but hard to study;
- evidence for pancreatic stellate cells as source of fibrosis in pancreas circumstantial;
- insulin, IGF and glucose influence pancreatic stellate cell survival in vitro.
Autoimmunity, Pancreatitis and Diabetes Mellitus
Shoichiro Tanaka (Yamanashi, Tokyo, Japan) discussed the characteristics of autoimmune pancreatitis, such as enlargement of the gland, narrowing of the main pancreatic duct, elevated IgG and IgG4, fibrotic changes involving lymphocyte and plasma cell infiltration of the exocrine pancreatic tissue, and improvement with steroid therapy [191, 192]. However, there is also insulitis and periinsulitis in patients with diabetes associated with autoimmune pancreatitis [193]. The pathological oral glucose test can be expected to be pathological before steroid therapy, but normalized after [194].
The Yamanashi group now favored a hypothesis that immune-mediated mechanisms could affect both the exocrine and endocrine pancreatic tissues through common autoantigens for exocrine and endocrine pancreatic tissues. They could show longitudinal changes of anti-HSP10 autoantibodies in patients with diabetes associated with autoimmune pancreatitis with a decrease after corticosteroids concomitant with decreased symptoms.
Tanaka concluded that:
- anti-HSP10 autoantibodies are frequently found in both patients with autoimmune pancreatitis and patients with fulminant type 1 diabetes;
- these findings suggest that autoimmuneresponse against HSP10 is possibly related with both exocrine pancreatic disease (pancreatitis) and endocrine pancreatic disease (diabetes mellitus).
Genes and Pancreatitis
Roland Pfützer (Mannheim, Germany) had the task to discuss the role of genetic predisposition to pancreatitis. Risk factors for chronic pancreatitis include:
- factors influencing initiation of acute pancreatitis;
- factors influencing environmental hazards (gene-environment interactions);
- factors influencing immune response.
Regarding the pathophysiology of chronic pancreatitis it may be interesting to think of which persons develop pancreatitis. Obviously most people with alcohol abuse will “never” develop pancreatitis although incidence of chronic pancreatitis and alcohol consumption is correlated. But several people with no apparent predisposition “do” develop pancreatitis. There are several possible risk factors:
- genetic predisposition;
- gene-gene interactions;
- environmental factors;
- gene-environment interactions.
There is a genetic predisposition in hereditary pancreatitis. This disease was first described by Comfort and Steinberg in 1952 [195]. The disease is autosomal dominant, with 80% penetrance, but is still a rare disease. The onset is usually early (about age 10 years), and with variable expressivity. The primary clinical manifestation is recurrent acute pancreatitis, which is clinically and pathologically indistinguishable from pancreatitis of other etiology. Affected individuals have a more than 50-fold increased risk of developing pancreatic cancer.
Today there are many described mutations in PRSS1 leading to hereditary pancreatitis (most probable there are also mutations in PRSS1 that do not lead to disease) (Table 11). However, there are today several known predisposing genes (Table 12).


It must be understood that this is not a blackor- white issue, but that there are different fenotypes, i.e. genetic variations in patients with chronic pancreatitis (Table 13).

SPINK1 (also called PSTI) is an intrapancreatic intracellular trypsin inhibitor that blocks activated trypsin by entering the specificity pocket. Normally, it binds only 20% of the amount of trypsin making it a perfect candidate as a pancreatitispredisposing gene. N34S is the most common mutation in pancreatitis. However, the mechanism of action is still unclear. The frequency of the mutation is about 1% in the general population [205].
PRSS2 is an AKA anionic trypsin. It makes up one third of total trypsin in pancreatic juice and it is increased in chronic alcohol use. This makes it another perfect candidate for pancreatitis associated mutation.
CFTR is a cAMP-sensitive chloride channel present in most epithelia. Loss of function leads to thickened secretion and loss of ability to hydrate macromolecules. CFTR is the driving force of bicarbonate secretion in the pancreas. Mutations in the CFTR gene are associated with “idiopathic”, familial and alcoholic chronic pancreatitis.
Regarding environmental factors, alcohol consumption and the incidence of chronic pancreatitis are positively correlated [209], but still only a small percentage of all heavy drinkers will develop chronic pancreatitis [210]. Moreover a strong association has been shown between smoking and pancreatitis in male patients, weaker association in females [211]. Smoking is significantly associated with pancreatitis, but not with cirrhosis [212]. There are also investigations showing that alcohol and smoking are independent risk factors for chronic pancreatitis [213] but with no difference between smoking prevalence in chronic pancreatitis and alcoholic controls [214].
There are also (older) studies on the HLAsystem and pancreatitis. AW23 and AW24 are associated with chronic calcifying pancreatitis of alcoholic origin [215] and HLA A and B antigens in chronic alcoholic pancreatitis [216, 217].
Pfützer concluded:
- pancreatic diseases have a strong genetic background;
- pancreatitis may be rather associated with genetic alterations initiating inflammatory events and conditions;
- the role of several pancreatitis associated mutations is poorly understood;
- CFTR mutations point to a role for ductal obstruction in chronic pancreatitis;
- polymorphisms and mutations in ethanol metabolizing genes point to the importance of gene-environment interactions;
- the role of HLA antigens in chronic pancreatitis warrants further investigation.
Gallstones, Obstruction and Pancreatitis
Hans-Ulrich Kloer (Giessen, Germany) discussed chronic pancreatitis and diabetes type 3c from the perspectives of pathophysiology of nutrition, digestion and metabolism. The mechanisms linking exocrine and endocrine pancreatic disease may be through chronic inflammatory destructive processes due to:
- genetic abnormalities facilitating and maintaining inflammation;
- autoimmune aggression against both exocrine and endocrine cells
- chronic exposure to toxic substances (alcohol, others), and infectious agents;
- obstruction of secretory flow triggered by the daily dietary and metabolic challenge of “Western“ lifestyle (fat, cholesterol) in, or without, conjunction with factors above.
Kloer called the metabolic syndrome leading to papillary stenosis “The Second Cholesterol Disease”. In estrogen dominance there is an anti-atherogenic lipid profile with increased HDL and low LDL. This protects from atherosclerosis but leads to a cholesterolsaturated (lithogenic) bile and microcrystal formation (1-3 mm) in the gallbladder (females more than males). Cholesterol crystals can induce chronic inflammation:
- crystals specifically activate the complement system;
- cholesterol crystals attract macrophages and induce the growth of smooth muscle cells;
- when implanted anywhere in the body, cholesterol crystals induce connective tissue (“scar tissue“) formation.
Papillary stenosis following bile stone passages can be regarded as a disease process analogous to plaque formation in the coronary arteries induced by cholesterol (“The Plaque of the Gastroenterologist“). The exocrine and endocrine pancreas is sensitive to obstruction at the papilla Vateri (papillary stenosis) leading to chronic pancreatitis. Repeated episodes of mild obstructive pancreatitis caused by increased ductal pressure permanent ductal damage.
This means that there are several perspectives both on prevention and treatment. For primary prevention the reduction of biliary cholesterol load and lithogenic index could be made by aggressive lipid lowering therapy (statins) and bile acid therapy. Statins reduce cholesterol saturation in bile (for example fluvastatin 80 mg for 12 weeks). For secondary prevention one must decrease obstructive damage to pancreatic ductal system with elimination of papillary stenosis as an obstructive mechanism.
Exocrine dysfunction has also been observed in obesity (n=559; Table 14):

What is then the reason for progression from impaired glucose tolerance (IGT) to fasting hyperglycemia (diabetes mellitus) in the metabolic syndrome? According to Kloer, it is as long as the pancreatic beta-cell produces enough insulin to overcome peripheral insulin resistance prolonged post-prandial and fasting hyperglycemia will not occur in the metabolic syndrome.
As there is clear evidence for the regeneration of islets of Langerhans from pancreatic small ducts in animals and humans in vivo and there is clear evidence for rodent and human pancreatic ductal cell “transformation“ into islets of Langerhans in culture, exocrine disease might result in disturbed islet regeneration.
It is now shown that fat digestion and absorption have a potentially beneficial effect on beta-cell function, but if a fat-rich diet can improve glucose control in non-insulin dependent diabetes mellitus (NIDDM) should be studied. It is also known that there is an increased secretion of insulin and GIP in chronic pancreatitis with pancreatic enzyme replacement [92]. There is still a decreased incretin-response in type 2 diabetes.
Kloer finished by stating how to diagnose and treat chronic (obstructive) pancreatitis and diabetes mellitus type 3c from a clinician’s perspective:
i) diagnosis of chronic (obstructive) pancreatitis:
- screening for low stool elastase in patients with the metabolic syndrome, gall stones, abdominal complaints and unintended weight loss;
- confirmation with imaging methods (MRI, endosonography, ERCP);
ii) treatment of chronic (obstructive) pancreatitis:
- enzyme replacement;
- anti-inflammatory treatment (antioxidants, omega-3 fatty acids);
- consider extensive papillotomy aiming at sufficient permanent relief of ductal obstruction and periductal inflammation in order to allow for improved function, decreased inflammation and regeneration;
iii) diagnosis of diabetes mellitus type 3c: - screening for low fecal elastase in all patients with glucose intolerance (all diabetic patients irrespective of previous classification, IGT);
- confirmation of chronic pancreatitis with imaging methods (MRI, endosonography, ERCP).
iv) treatment of diabetes mellitus type 3c:
- pancreatic enzyme replacement in all diabetics with fecal elastase less than 100 μg/g;
- emphasis on stimulation of the incretin axis (DPP-4 inhibitors) in all NIDDM patients;
- possibly combination of incretin stimulation with a relatively fat-rich (omega-3 PUFA) diet in NIDDM;
- consider extensive papillotomy aiming at sufficient permanent relief of ductal obstruction in order to allow for regeneration of the ductal cell system (recovery of endocrine precursor cells).
After two days packed with lectures and discussions the audience obviously felt that there are still more questions to be solved concerning interactions between exocrine and endocrine pancreas - but we can outline the issues better now (the fog is lifting). The meeting was only adjourned and when it is hopefully reopened in about three years time there will probably be more answers (and even more questions).
The organizers want to thank all invited participants (Table 15) for their valuable contributions
