Katerina Vaporidi,
Nectaria Xirouchaki and
Dimitris Georgopoulos*
University of Crete, School of Medicine, Department of Intensive Care, University
Campus of Heraklion, Crete, 70010, Greece
- *Corresponding Author:
- Dimitris Georgopoulos
University of Crete, School of Medicine
Department of Intensive Care
University
Campus of Heraklion
Crete, 70010, Greece.
Tel: 302810392363
Fax: 302810392409
E-mail: georgop@uoc.gr
Received date: May 22, 2017; Accepted date: May 28, 2017; Published date: May 31, 2017
Citation: Vaporidi K, Xirouchaki N, Georgopoulos D. Should We Care about
Driving Pressure during Assisted Mechanical
Ventilation? J Intensive & Crit Care 2017,
3:2.
Keywords
Control of breathing; Reflex and chemical feedback; Transpulmonary
pressure; Ventilator induced lung injury
Introduction
During passive mechanical ventilation, at absence of dynamic
hyperinflation, driving pressure of respiratory system (ΔP) is
defined as static end-inspiratory plateau pressure (Pplat) minus
external positive end-expiratory pressure (PEEP), and equals
tidal volume (VT) to respiratory system compliance (Crs) ratio.
ΔP essentially reflects the extent of lung stretch during tidal
breathing. Thus, ΔP may reflect better than Pplat or VT, expressed
as ml/kg of ideal body weight, the alveolar distortion during
inspiration because it takes into account the available aerated
lung volume. Indeed, a large retrospective study in patients with
acute respiratory distress syndrome (ARDS) identified ΔP as the
main determinant of ventilator-induced lung injury (VILI), and
the ventilator parameter most strongly related to mortality,
particularly at ΔP values >14 cm H2O [1].
The association of ΔP with mortality in ARDS patients was
confirmed in another large observational study [2]. Other
studies also showed that high ΔP may detect lung overstress
[3] and be associated with high morbidity [4]. However, we
should notice that ΔP is dissipated to counterbalance both the
change in transpulmonary pressure (ΔPlung) and that of chest
wall (ΔPcw), the first being the key variable for lung damage.
Since ΔPlung calculation requires an esophageal catheter insertion
for recording esophageal pressure, a procedure not easily
applicable for every day practice, ΔP is used as surrogate of ΔPlung
(ΔP=ΔPlung+ΔPcw).
Although ΔP, as marker of VILI, has been exclusively studied in
patients under controlled mechanical ventilation, there is no
reason to believe that the potential harmful effects of high ΔP
(due to high ΔPlung) are present only during passive ventilation.
During assisted mechanical ventilation the ventilator and the
respiratory muscles may be considered as pressure generators
arranged in series, and thus during inspiration the total pressure
applied to respiratory system at any time t (PTOT(t)) is the sum of
airway pressure (pressure provided by the ventilator, Paw(t)) and
pressure developed by inspiratory muscles (Pmus(t)). This total
pressure is dissipated to offset elastic and resistive pressures
according to the equation of motion:

Where V and V’ are volume above end-expiratory lung volume
and flow, respectively. Notice that Equation 1 as it stands, is valid
if intrinsic PEEP is zero (no dynamic hyperinflation). Obviously
during assisted modes ΔP is the pressure dissipated to offset
the increase in elastic recoil pressure of respiratory system
(ΔPel) due to VT (ΔPel=ΔP=VT/Crs). It becomes apparent that, as
opposed to controlled mechanical ventilation, during assisted
mechanical ventilation ΔP is partially depended and, to some
extent, regulated by patient effort (Pmus).
May ΔP reach injurious levels during assisted ventilation? ΔP is
high, when VT is high (i.e., as a result of high PTOT=Paw+Pmus)
and/or when Crs is low. Regarding Crs, one could hypothesize
that patients having more severe lung injury and thus lower Crs,
would be ventilated on controlled modes, and assisted modes
would be reserved for patients with better lung mechanics,
limiting thus the risks of high ΔP. However, although a patient
with severe ARDS would be more likely sedated and ventilated in
controlled mode, at least initially, no studies or guidelines have
indicated switching between controlled and assisted ventilation
based on lung mechanics. A second hypothesis could be that the
system of control of breathing (non-functioning during passive mechanical ventilation) would prevent the development of
high distending pressures. Indeed, the mechanoreceptors of
the respiratory system sense the degree of lung stretch and
tend to prevent over-distension through reflex mechanisms.
The Hering-Breuer inspiratory-inhibitory reflex inhibits
inspiratory activity and associated increase in lung volume,
when a threshold lung stretch is reached during inspiration [5].
In addition, if high distending pressure results in high VT this
may decrease PaCO2 which via chemical feedback mechanism
lowers Pmus limiting thus ΔP. Nevertheless, the effectiveness of
these protective mechanisms is heavily depended on the mode
of mechanical ventilation and ventilator settings as Equation 1
dictates (i.e., Paw). In addition, assist volume control or pressure
supports modes cancel or limit the ability of the patient effort
to modulate VT. Also the proper function of these protective
feedback mechanisms may be overridden by other stimuli that
increase the respiratory drive of critically ill patients. Indeed it
has been shown in experimental settings that metabolic acidosis
induces sufficient hyperventilation to develop lung injury [6].
In critically ill patients, conditions increasing respiratory drive,
such as hypercapnia, metabolic acidosis, delirium, fever, ongoing
sepsis are often present and could result in overriding the
protective mechanisms of control of breathing [7]. It is therefore,
possible that, in the presence of impaired lung mechanics and
high respiratory drive, injurious ΔP may develop during assisted
ventilation, promoting ventilator-induced lung injury.
Although ΔP is an important variable during assisted modes,
its calculation requires either Pmus or valid Crs measurements.
Pmus measurement can be obtained by recording Pes, in which
case, however, it is easier to measure ΔPlung. On the other hand,
valid Crs is difficult to be obtained during assisted modes of
support such as volume-assist, pressure support or neutrally
adjusted ventilator assist (NAVA), because it necessitates passive
conditions during the measurement of Pplat. Currently the only
assisted mode that permits semi-continuously valid measurement
of Crs (and thus of ΔP) is proportional assist ventilation with
adjustable gain factors (PAV+), a mode that permits the patients
to select their own breathing pattern as dictated by the control
of breathing mechanisms. With this mode, the ventilator applies
a 300-msec end-inspiratory occlusion randomly every 4-7
breaths and measures airway pressure (PplatPAV+) at the end of
this brief occlusion. Crs is calculated as the VT/(PplatPAV+-PEEP)
ratio. It has been shown that there is no residual post-inspiratory
activity at the point of Pplat PAV+ measurement and because it
is measured very close to the end of inspiratory flow (which is
mainly determined by the end of neural inspiration), it is a good
approximation of the true elastic recoil pressure at end inspiration
in un-occluded breaths [8]. We have recently reported data on
ΔP during assisted ventilation [9] by analyzing 108 patients (64
were recovering from ARDS) ventilated on PAV+ for 48h after
switching from controlled ventilation [10]. When patients were
switched from passive ventilation to PAV+ they controlled ΔP
to low levels (<15 cm H2O in 90% of measurements) without
constraining VT to narrow limits. It seems that in critically ill
patients meeting certain criteria, the control of breathing system
was adept at protecting the lungs by preventing high ΔP, while not unnecessarily restricting VT when this had no protective value
(Figure 1). However, in two patients having very low values of
Crs, constantly high ΔP (≥ 15 cm H2O) was observed. In addition,
unpublished data from our group indicate that approximately
10% of patients during assisted ventilation on PAV+ may at some
point, exhibit high ΔP, associated with low CRS. Notwithstanding
that ΔP is a surrogate of ΔPlung, these data indicate that in some
patients, especially those with impaired Crs, the protective
mechanisms of control of breathing may be overridden by high
respiratory drive due to a variety of causes. Monitoring ΔP during
assisted mechanical ventilation may identify these patients who
are at risk of lung injury and influence decision making.
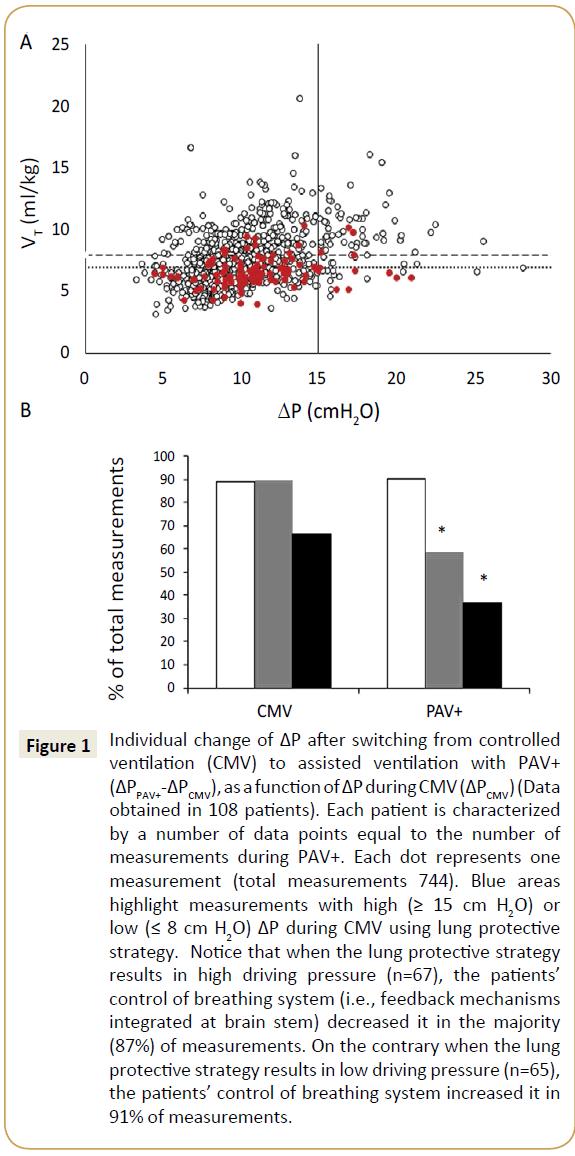
Figure 1: Individual change of ΔP after switching from controlled
ventilation (CMV) to assisted ventilation with PAV+
(ΔPPAV+-ΔPCMV), as a function of ΔP during CMV (ΔPCMV) (Data
obtained in 108 patients). Each patient is characterized
by a number of data points equal to the number of
measurements during PAV+. Each dot represents one
measurement (total measurements 744). Blue areas
highlight measurements with high (≥ 15 cm H2O) or
low (≤ 8 cm H2O) ΔP during CMV using lung protective
strategy. Notice that when the lung protective strategy
results in high driving pressure (n=67), the patients’
control of breathing system (i.e., feedback mechanisms
integrated at brain stem) decreased it in the majority
(87%) of measurements. On the contrary when the lung
protective strategy results in low driving pressure (n=65),
the patients’ control of breathing system increased it in
91% of measurements.
Conclusion
The recognition of VILI changed the practice of mechanical
ventilation. The pursue of optimal ventilator settings to reduce
lung stress and injury is an on-going journey, and the setting of
VT to 6 ml/kg was just the beginning. Targeting ΔP as means to
minimize lung injury appears to be reasonable, emphasized by the fact that our brain seems to care more for the ΔP than for
VT. Identifying the appropriate target for driving pressure will
be challenging, as the benefits from protection from VILI have
to balance against the costs of interventions to reduce ΔP, such
as sedation to reduce respiratory drive. To this end, prospective
studies evaluating ΔP in both controlled and assisted ventilation
are urgently needed.
References
- Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, et al. (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372: 747-755.
- Laffey JG, Bellani G, Pham T, Fan E, Madotto F, et al. (2016) Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: The LUNG SAFE study. Intensive Care Med 42: 1865-1876.
- Chiumello D, Carlesso E, Brioni M, Cressoni M, (2016) Airway driving pressure and lung stress in ARDS patients. Crit Care (London, England) 20: 276.
- Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, et al. (2016) Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: A meta-analysis of individual patient data. Lancet Resp Med 4: 272-280.
- Clark FJ, von Euler C, (1972) On the regulation of depth and rate of breathing. J Physiol 222: 267-295.
- Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y, (2013) The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 41: 536-545.
- Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, et al. (2016) Control of respiratory drive and effort in extracorporeal membrane oxygenation patients recovering from severe acute respiratory distress syndrome. Anesthesiology 125: 159-167.
- Younes M, Webster K, Kun J, Roberts D, Masiowski B (2001) A method for measuring passive elastance during proportional assist ventilation. Am J Resp Crit Care Med 164: 50-60.
- Georgopoulos D, Xirouchaki N, Tzanakis N, Younes M (2016) Driving pressure during assisted mechanical ventilation: Is it controlled by patient brain? Resp Physiol Neurobiol 228: 69-75.
- Xirouchaki N, Kondili E, Vaporidi K, Xirouchakis G, Klimathianaki M, et al. (2008) Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 34: 2026-2034.