Keywords
Articaine HCl; Epinephrine; Sodium dodecyl sulphate; Carbon paste;
Voltammetry; Bulk; Urine
Introduction
Articaine HCl is a white or almost white crystalline powder, freely
soluble in water, methanol and ethanol [1]. It is an intermediatepotency,
short-acting amide local anesthetic with a fast
metabolism due to an ester group in its structure. It is effective
with local infiltration or peripheral nerve block in dentistry, when
administered as a spinal, epidural, ocular, or regional nerve block,
or when injected intravenously for regional anesthesia. Local
anesthetic drugs are administered to the areas around the nerves
to be blocked as the skin, subcutaneous tissues, retrobulbar,
intrathecal, and epidural spaces. Articaine has a half-life of 60
minutes after entering the circulation and is quickly metabolized
via hydrolysis into its inactive metabolite articainic acid, which is
partly metabolized in the kidney into articainic acid glucuronide.
Seventy-five percent of articainic acid is excreted unchanged; the
rest is glucuronidated by the kidneys before excretion [2]. It is
an amino-amide anesthetic having a thiophene, rather than a
benzene ring, as well as an additional ester group that is subject to
metabolism by plasma esterases. It has a widespread popularity
in dental anesthesia, where it is generally considered to be more
effective, and possibly safer, than lidocaine, the prior standard [3].
Epinephrine greatly increases the duration of the local anesthesia by producing vasoconstriction at the site of injection. This allows
the local anesthetic to persist at the injection site before being
absorbed into the systemic circulation [4].
Literature survey reveals that several different analytical
techniques have been developed for the determination of
including high performance liquid chromatographic methods [5-7], thin layer chromatographic methods [8], gas chromatographic
methods [9] and spectrophotometric methods [10] for articaine
HCl and high performance liquid chromatographic methods
[11-15], thin layer chromatographic methods [16,17], gas
chromatographic methods [18,19], spectrophotometric methods
[20-24] and electrochemical methods [25-30] for epinephrine.
Carbon based electrodes are currently in widespread use in
electroanalytical chemistry, because of their broad potential
window, low cost, rich surface chemistry, low background current
and chemical inertness. Carbon paste electrode (CPE) has some special characteristics and benefits such as the ease of surface
renewal, individual polarizability, and easy to apply modifications.
The disadvantage of CPE is the tendency of the organic binder to
dissolve in solutions containing an appreciable fraction of organic
solvent [31].
In this study articaine HCl and epinephrine were determined
simultaneously using differential pulse voltammetry (DPV) at
carbon paste electrode in bulk, pharmaceutical formulations and
human urine.
Materials and Methods
Experimental preparations
Pure and market samples: Articaine HCl and epinephrine
bitartrate were kindly supplied by Inibsa Laboratories, Spain.
Their purity was 100% as stated by the supplier.
Artinibsa ampoules; batch no. K-3, labeled to contain 40 mg
Articaine HCl and 0.01 mg Epinephrine per ampoule, product of
Inibsa Laboratories, Spain.
Standard solutions: Standard solutions of articaine HCl and
epinephrine bitartarate were prepared in methanol. For cyclic
voltammetry (CV) 1.0 x 10−2 mol L−1 solutions were prepared by
dissolving 32.1 mg of articaine HCl and 33.3 mg of epinephrine
bitartarate, each in 10 mL volumetric flask and diluted with
methanol. For DPV, one mL of each 1.0 × 10−2 mol L−1 solution was
taken and diluted to 10 mL with the same solvent to obtain 1.0 ×
10−3 mol L−1 working solution.
Chemicals and reagents: All reagents used were of analytical
grade and solvents were of spectroscopic grade. Distilled water
was used throughout the work.
(i) Methanol, HPLC grade (Fischer Chemical, UK).
(ii) Sodium hydroxide (Qualikems fine chemical Pvt. Ltd).
(iii) Sodium dodecyl sulphate (SDS) (Sigma-Aldrich, Germany).
(iv) Dioctyl sodium sulfosuccinate (DSS) (Sigma-Aldrich,
Germany).
(v) Hexane Sulfonic acid sodium salt monohydrate (HSA)
(Sharlab S.L., Spain).
(vi) Potassium dihydrogen o-phosphate (El Nasr
Pharmaceutical Chemicals, Egypt).
(vii) Phosphoric acid (Sigma-Aldrich, Germany).
(viii) Britton-Robinson (BR) buffer was prepared by mixing
0.04 mol L−1 of phosphoric acid (Sigma-Aldrich), acetic acid
(Loba Chemie Co., India) and boric acid (Sigma-Aldrich).
Buffer solutions were adjusted with the appropriate amount of
0.2 mol L−1 NaOH (Qualikems fine chemical Pvt. Ltd) to get the
desired pH 7-11 [32].
Phosphate buffer was prepared by adding 34.7 mL of 0.2 mol L−1
NaOH to 50 mL of 0.2 mol L−1 monobasic potassium phosphate
solution (prepared by dissolving 27.22 g monobasic potassium
phosphate in water and dilute with water to 1000 mL) and
complete to 200 mL with water [32].
Instruments: All voltammetric measurements were carried out
using a computer-driven analytical electrochemical workstation
(model AEW2) with ECProg3 electrochemistry software (Sycopel,
England) in combination with a three-electrode configured C-3
stand. The working electrode was a modified glassy carbon disc
electrode (MF-2010, BAS model), the reference electrode Ag/
AgCl/ 3 mol L−1 NaCl (MW-2063, BAS model) and a platinum wire
counter electrode (MW-1032, BAS model). A digital pH-meter
(Jenway pH meter, UK) with combined glass electrode was used to
carry out the pH measurements. All electrochemical experiments
were performed at an ambient temperature of 25 ± 0.2°C.
Procedures
Preparation of working electrode: In a mortar 0.5 g graphite
powder and 0.3 mL paraffin oil were mixed thoroughly with
a pestle. The paste was packed into the hole of the electrode
body and smoothed on a filter paper until it acquired a shiny
appearance; the paste was carefully removed and replaced by a
new one after each measurement [33].
Linearity: Aliquots of working standard articaine HCl and
epinephrine solutions (1.0 × 10−3) were added separately to the
electrolytic cell containing 5 mL of Britton-Robinson buffer of pH
7 and 40 μL of 1.0 × 10−2 mol L−1 SDS, the solutions were stirred for
5 s at open circuit conditions, voltammetric analyses were carried
out and voltammograms were recorded at a scan rate 20 mV s−1.
Calibration curve was constructed by plotting anodic peak current
against drug concentrations in molarity; and the regression
equation was computed.
Accuracy and precision: Three different concentrations covering
the linearity range of articaine HCl and epinephrine were analyzed
in triplicates within the same day for intraday and for three
successive days for interday using the procedure detailed under
linearity part. Accuracy was calculated as precision (Relative
standard deviation (%RSD)).
Application to pharmaceutical formulations
An aliquot (0.8 mL) containing 32.1 mg articaine HCl and 0.0144
mg epinephrine bitartarate was transferred to 10 mL volumetric
flask, 33.286 mg pure standard epinephrine bitartarate was
transferred to the volumetric flask and volume completed with
methanol to obtain 1.0 × 10−2 mol L−1 solution of each drug. One
mL of the prepared 1.0 × 10−2 mol L−1 solution was taken and
diluted to 10 mL with the same solvent to obtain 1.0 ×10−3 mol L−1
working solution to be analyzed by the proposed electrochemical
method using the procedure detailed under linearity part. The
drug concentrations were calculated from the corresponding
regression equation; the proposed method was further validated
by using the standard addition technique.
Analysis of articaine HCl and epinephrine in urine: For the analysis
of articaine HCl and epinephrine in urine, 1.0 mL of urine was
mixed with 9.0 mL BR buffer of pH 7.0, then successive additions
of 1.0 × 10−3 mol L−1 working standard solutions (covering the
linearity range) were added to the voltammetric cell containing
5.0 mL of the previously diluted urine.
Results and Discussion
Electrochemical oxidation of articaine HCl
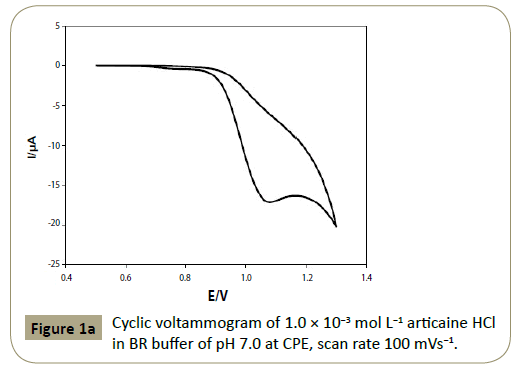
The reversibility of the oxidation process of articaine HCl at CPE
was studied by CV, the cyclic voltammogram showed one well
defined anodic peak in BR buffer of pH 7.0 (anodic peak current
(I)=19.58 μA at 1.095 V), and the reverse scan showed no cathodic
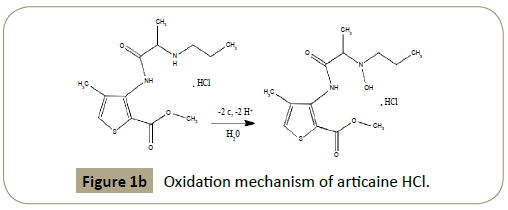
peak indicating that articaine HCl oxidation is irreversible (Figure 1a). The proposed oxidation mechanism was shown in Figure 1b.
Figure 1a: Cyclic voltammogram of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl in BR buffer of pH 7.0 at CPE, scan rate 100 mVs-1.
Figure 1b: Oxidation mechanism of articaine HCl.
Optimization of experimental conditions
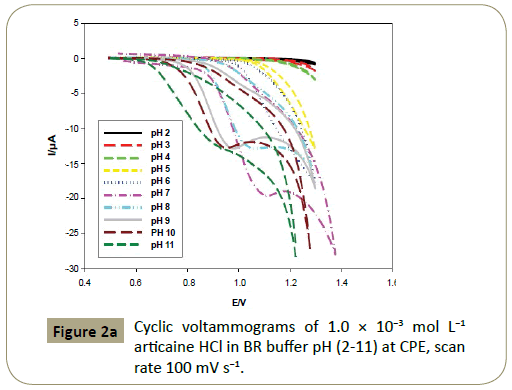
Effect of pH: CV at CPE and scan rate of 100 mV s−1 was used to
investigate the effect of pH upon the oxidation of articaine HCl.
4.5 mL of BR buffer (2-11) and 0.5 mL of 1.0 × 10−2 mol L−1 articaine
HCl standard solution were added to the electrochemical cell, and
the respective voltammetric response was recorded (Figure 2a).
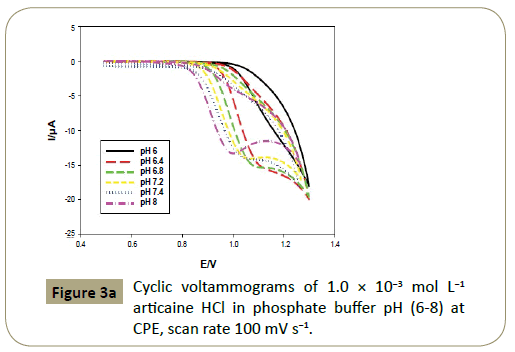
This experiment was repeated using phosphate buffer (Figure 3a)
to obtain the optimum buffer with the optimum pH value.
Figure 2a: Cyclic voltammograms of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl in BR buffer pH (2-11) at CPE, scan rate 100 mV s-1.
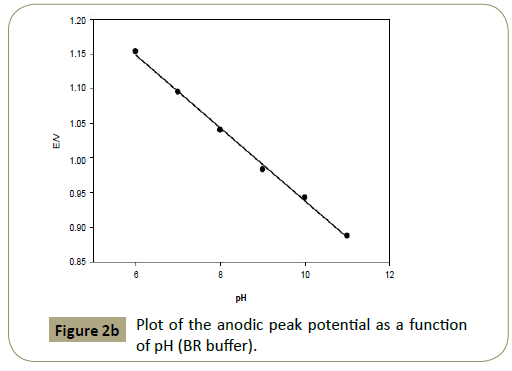
Figure 2b: Plot of the anodic peak potential as a function of pH (BR buffer).
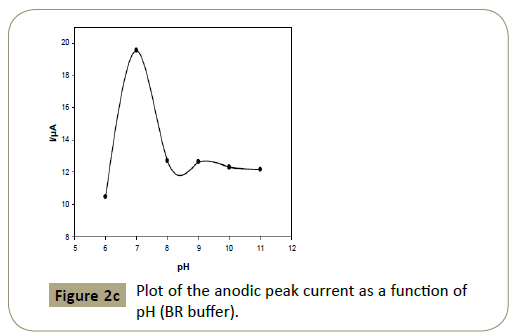
Figure 2c: Plot of the anodic peak current as a function of pH (BR buffer).
Figure 3a: Cyclic voltammograms of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl in phosphate buffer pH (6-8) at CPE, scan rate 100 mV s-1.
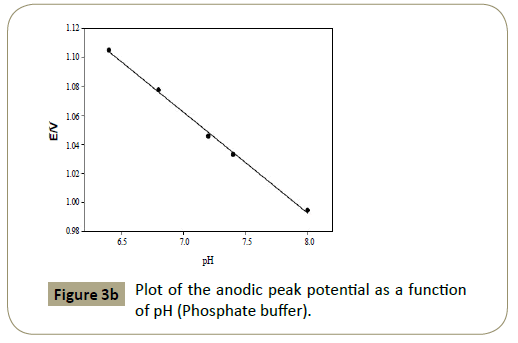
Figure 3b: Plot of the anodic peak potential as a function of pH (Phosphate buffer).
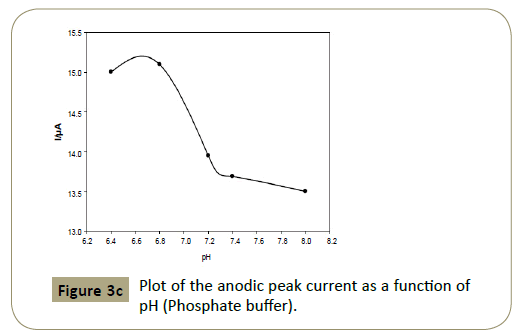
Figure 3c: Plot of the anodic peak current as a function of pH (Phosphate buffer).
Figures 2c and 3c show that the anodic peak potential (E) was
shifted negatively by increasing pH indicating that articaine HCl
oxidation at CPE is pH dependant and those protons are involved
in the reaction. Figures 2c and 3c show that higher peak current
was achieved using BR buffer of pH 7.0.
The anodic peak current at pH 7 (BR buffer) was found 19.58
μA and at pH 7.2 (Phosphate buffer) was 15.10 μA. The linear
relations between pH and peak potential (E) observed in the pH
range 7-11 (BR) buffer, and 6.4-8.0 phosphate buffer was used
to apply Nernest equation as shown in Figures 2b and 3b with
a regression equation: E(V)=−0.053 pH+1.465, R2 (correlation
coefficient=0.9974 and E(V)=−0.0695 pH+1.548, R2=0.9977 in
case of BR and phosphate buffers, respectively. Applying Nernst
equation using the formula ΔEp/ΔpH (Slope)=0.059x/n, where
x and n is the number of protons and electrons involved in
the reaction, respectively. It can be concluded that number of
electrons transferred is equal to the number of protons, thus n=x
[34,35].
Effect of surfactant: A surface-active agent (surfactant) is
one which tends to accumulate at a surface or interface. A
prerequisite for surfactants to be surface active is the property of these molecules to adsorb at the interface between bulk phases,
such as air and water, oil and water or electrode and solution.
The distinct structural feature of a surfactant is the hydrophilic
region of the molecule or the polar head group which may be
positive, negative, neutral or zwitterionic and the hydrophobic
region or the tail that consists of one or more hydrocarbon
chains, usually with 6-22 carbon atoms, thus they are also called
amphiphiles, i.e., compounds having both polar and nonpolar
regions in their molecules. Depending on the chemical structure
of the hydrophilic moiety bound to the hydrophobic portion, the
surfactant may be classified as cationic, anionic, non-ionic or
zwitterionic.
Two important properties of surfactants, adsorption at
interface and aggregation into supramolecular structures are
advantageously used in electrochemistry. Surfactants can modify
and control the properties of electrode surfaces (Figure 4) [36].
Figure 4: Side views of surface micelles or cylinders.
Surfactants were widely used in electroanalytical chemistry
to improve sensitivity and selectivity and were applied for
determination of potassium ferricyanide and dopamine [37],
diethylstilbestrol [38], dopamine, uric acid and ascorbic acid
[39], uric and ascorbic acids [40], thyroxine [41], moexipril
hydrochloride [33], cefdinir [42], drotaverine hydrochloride [43]
and atorvastatin [44].
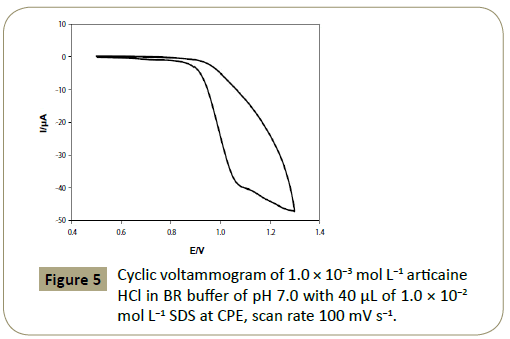
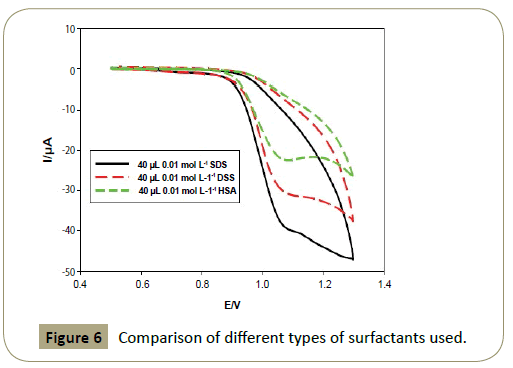
The anodic peak current was found to be 19.58 μA at 1.095 V in
BR buffer of pH 7.0 at CPE (Figure 1a), and 39.28 μA at 1.065 V in presence of 40 μL of 1.0 x 10−2 mol L−1 SDS (Figures 5 and 6) which
enhances the current value and showing catalytic effect.
Figure 5: Cyclic voltammogram of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl in BR buffer of pH 7.0 with 40 μL of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-2 mol L-1 SDS at CPE, scan rate 100 mV s-1.
Figure 6: Comparison of different types of surfactants used.
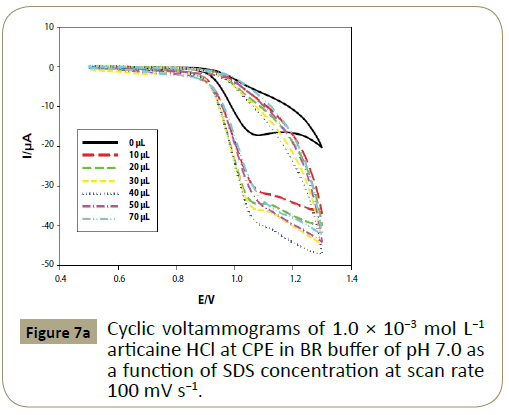
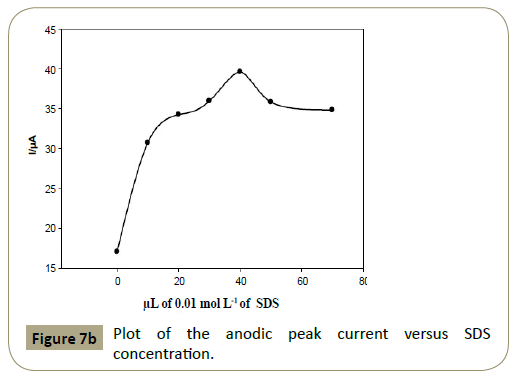
Effect of SDS concentration: The anodic peak current of articaine
HCl increased gradually upon increasing the concentration of
SDS, however it decreased when SDS concentration exceeded 8.0
×10−5 mol L−1 (40 μL of 1.0 × 10−2 mol L−1 SDS), which was chosen
as the optimum concentration of SDS. The relationship between
anodic peak current of articaine HCl and SDS concentration was
illustrated in Figures 7a and 7b.
Figure 7a: Cyclic voltammograms of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl at CPE in BR buffer of pH 7.0 as a function of SDS concentration at scan rate 100 mV s-1.
Figure 7b: Plot of the anodic peak current versus SDS concentration.
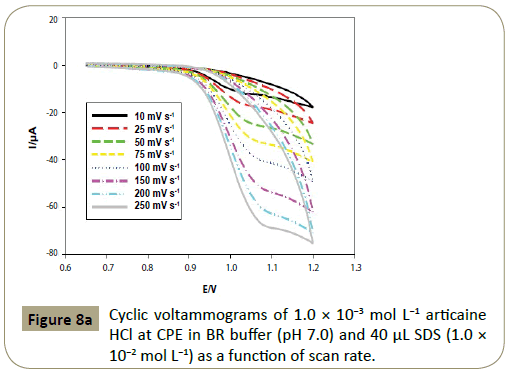
Effect of scan rate: The effect of different scan rates (υ) ranging
from 10 to 250 mV s−1 on the cyclic voltammetric response
of articaine HCl in BR buffer (pH 7.0) was investigated, with
increasing scan rates, the anodic peak was slightly shifted to
the positive potential direction and the peak current increased
remarkably with increasing scan rates (Figure 8a). It was found
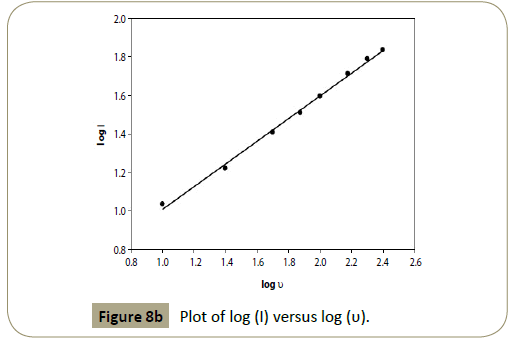
that the logarithm of anodic peak current (log I) is linear to the logarithm of scan rate (log υ), with the linear regression equation
as log I=0.59 log υ+0.421, R2=0.9966. From the value of the slope,
0.59, it can be deduced that the electrochemical oxidation process
of articaine HCl at CPE is diffusion controlled process with an
adsorption contribution (Figure 8b) [45].
Figure 8a: Cyclic voltammograms of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl at CPE in BR buffer of (pH 7.0) and 40 μL SDS (1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-2 mol L-1) as a function of scan rate.
Figure 8b:Plot of log (I) versus log (υ).
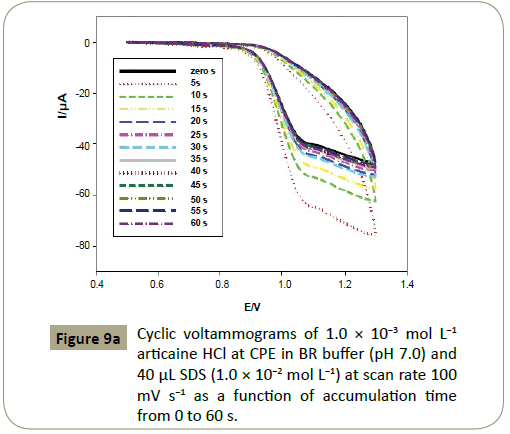
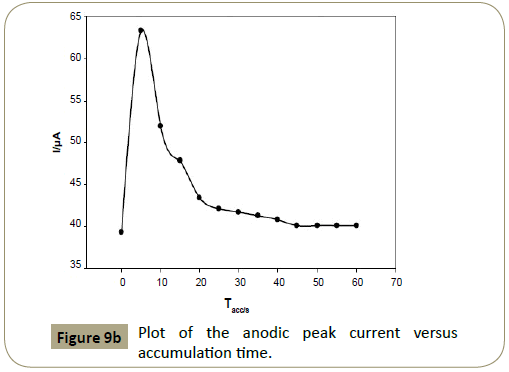
Effect of accumulation time: It was found that the peak current depended on the accumulation time (Tacc) of 1.0 ×10−3 mol L−1
articaine HCl at CPE in BR buffer (pH 7.0) and 40 μL SDS (1.0× 10−2 mol L−1). Sharp increase was observed at 5 s reaching its
maximum value, and then decreased with increasing time. 5 s was chosen as the optimum Tacc for determination of articaine
HCl (Figures 9a and 9b).
Figure 9a: Cyclic voltammograms of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl at CPE in BR buffer (pH 7.0) and 40 μL SDS (1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-2 mol L-1) at scan rate 100 mV s-1 as a function of accumulation time from 0 to 60 s.
Figure 9b: Plot of the anodic peak current versus accumulation time.
Simultaneous determination of articaine HCl and
epinephrine
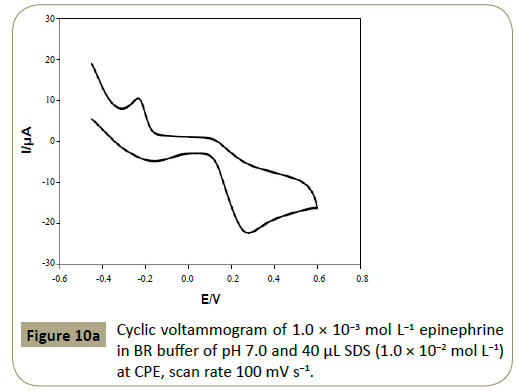
Figure 10a shows the cyclic voltamogram of 1.0 ×10−3 mol L−1
epinephrine at CPE in BR buffer (pH 7.0) containing 40 μL SDS (1.0
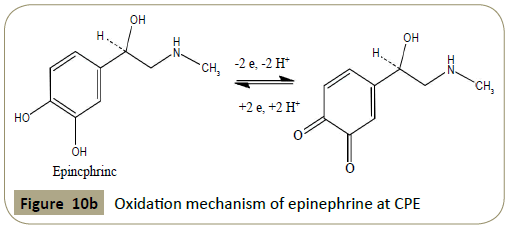
× 10−2 mol L−1). Figure 10b exhibits the oxidation mechanism of
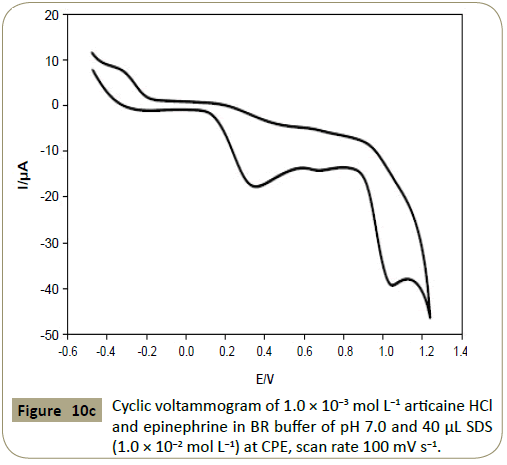
epinephrine [34]. Figure 10c presents the cyclic voltammogram
of 1.0 × 10−3 mol L−1 articaine and epinephrine at CPE using the
same conditions referring to well defined separate peaks of
articaine and epinephrine were obtained, therefore these drugs
can be determined simultaneously.
Figure 10a: Cyclic voltammogram of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 epinephrine in BR buffer of pH 7.0 and 40 μL SDS (1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-2 mol L-1) at CPE, scan rate 100 mV s-1.
Figure 10b: Oxidation mechanism of epinephrine at CPE
Figure 10c: Cyclic voltammogram of 1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-3 mol L-1 articaine HCl and epinephrine in BR buffer of pH 7.0 and 40 μL SDS (1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-2 mol L-1) at CPE, scan rate 100 mV s-1.
Method validation
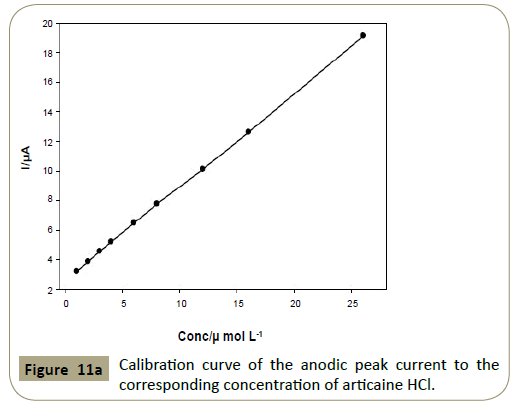
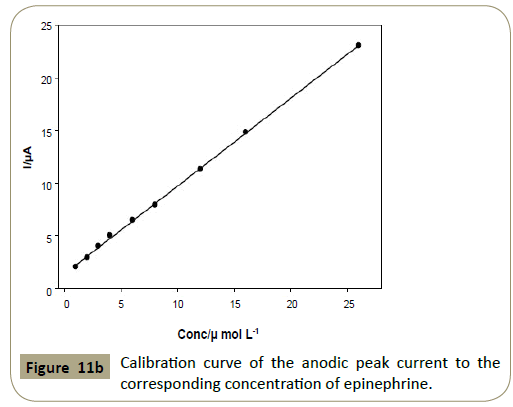
Linearity: Linear relationships were found between the peak
currents and concentrations of the two drugs in the range of
1.0 × 10−6- 2.6 × 10−5 mol L−1 (0.320-8.34 μg mL−1) for articaine
HCl and epinephrine using the proposed method as shown in
Figure 11. The mean percentage recoveries were of 98.1 ± 1.96
and 98.2 ± 1.94 for articaine HCl and epinephrine, respectively.
Figure 11a: Calibration curve of the anodic peak current to the corresponding concentration of articaine HCl.
Figure 11b: Calibration curve of the anodic peak current to the corresponding concentration of epinephrine.
The proposed method is more sensitive than spectrophotmetric
method for articaine HCl 10-70 μg/mL [10], spectrophotometric
methods for epinephrine: 2-20 mg mL−1 [20], 1.5-30, 3.0-30, and
1.5- 25 μmol L−1 [21], 0.4-12.8 mg mL−1 [22], 1.143-142.90 μg mL−1 [23], and 1.8-35.3 μg mL−1 [24], and electrochemical methods
for epinephrine: 3.0-175.0 μmol L−1 [26], and 1.0 × 10−5 to 1.0 ×
10−3 mol L−1 [29]. The regression parameters were computed and
presented in Table 1.
| Parameter |
Articaine HCl |
Epinephrine |
| Linearity range (mol L-1) |
1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-6- 2.6 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-5 |
1.0 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-6- 2.6 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-5 |
| Slope ± SD* |
0.631 ± 0.57 |
0.834 ± 0.49 |
| Intercept ± SD* |
2.637 ± 0.055 |
1.370 ± 0.013 |
| R2 |
0.9998 |
0.9995 |
| LOD (mol L-1) |
2.88 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-7 |
5.10 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-8 |
| LOQ (mol L-1) |
8.72 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-7 |
1.56 ÃÆÃâÃâ ââ¬â¢ÃÆââ¬Å¡Ã¢ââ‰â¬Â 10-7 |
*Average of three determinations at each level
Table 1: Regression parameters for the determination of articaine HCl and epinephrine by proposed electrochemical method.
LOD and LOQ: The limits of detection (LOD) and of quantification
(LOQ) were determined according to ICH [46] using the standard
deviation of multiple blank samples and the slope of the
calibration curve. The limits of detection and quantification were found 2.88 × 10−7 mol L−1 and 8.72 × 10−7 mol L−1 for articaine HCl
and 5.10 × 10−8 mol L−1 and 1.56 × 10−7 for epinephrine, respectively
(Table 2).
|
Parameter |
Articaine HCl |
Epinephrine |
|
%RSD* |
%R* |
%RSD* |
%R* |
| pH 6.8 |
0.98 |
100.8 |
0.53 |
99.15 |
| pH 7.2 |
0.14 |
98.9 |
0.38 |
99.70 |
| 38 μ |
0.63 |
101.3 |
1.21 |
100.35 |
|
42 μ |
1.52 |
100.8 |
0.85 |
101.6 |
*Average of three determinations at each level.
Table 2: Robustness results of the proposed DPV method.
Robustness
The robustness of proposed DPV method using 6.0 × 10−6 mol L−1
of the two drugs was evaluated by variation in the concentration
of SDS and pH of BR buffer. The relative standard deviation (%RSD)
and Recover (%R) values listed in Table 3 indicate that the DPV
method was not affected by deliberate changes in the optimum
parameters of the method.
| Procedure |
Taken (μmol L-1) |
Intraday |
Interday |
Found
(μmol L-1) |
%R* |
%RSD* |
Found
(ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâõmol L-1) |
%R* |
%RSD* |
| Articaine HCl |
2 |
2.03 |
101.5 |
1.03 |
2.01 |
100.5 |
1.49 |
| 8 |
7.94 |
99.3 |
1.1 |
8.07 |
100.9 |
1.1 |
| 12 |
12.04 |
100.3 |
1.25 |
12.2 |
101.7 |
0.23 |
| Epinephrine |
1 |
0.991 |
99.1 |
0.86 |
0.983 |
98.3 |
1.12 |
| 8 |
8.04 |
100.5 |
1.41 |
8.12 |
101.5 |
0.68 |
| 12 |
12.15 |
101.3 |
0.58 |
11.8 |
98.3 |
1.78 |
*Average of three determinations at each level.
Table 3: Intraday and Interday accuracy and precision for the simultaneous determination of articaine HCl and epinephrine by the proposed electrochemical method.
Accuracy and precision
Intraday and interday accuracy (%R) ranged from 99.3% to 101.7%
and precision (%RSD) from 0.23% to 1.49% for articaine HCl and
from 98.3% to 101.5% and from 0.58% to 1.78% for epinephrine
(Table 4).
|
Articaine HCl |
Epinephrine |
|
Mean ± RSD |
Standard addition |
Mean ± RSD |
Standard addition |
Artinibsa®
carpules |
Claimed
taken
(μmol L-1) |
Pure
added
(μmol L-1) |
%R of
added |
Artiniba®
carpules |
Claimed
taken
(μmol L-1) |
Pure
added
(μmol L-1) |
%R of
added |
| 6.0 |
2 |
100.6 |
1.0 |
1 |
99.8 |
| 4 |
100.6 |
3 |
98.8 |
| 6 |
100.3 |
5 |
98.8 |
| 8 |
101.3 |
7 |
98.5 |
| 12 |
99.4 |
11 |
99.6 |
| |
18 |
99.9 |
|
15 |
98.2 |
| |
|
|
25 |
100.2 |
| Mean ± RSD |
100.4 ± 0.65 |
Mean ± RSD |
99.2 ± 0.71 |
Table 4: Application of standard addition technique for the simultaneous determination of articaine HCl and epinephrine in Artinibsa® carpules by the proposed electrochemical method.
Application to pharmaceutical formulation
The proposed method was applied successfully for the
determination of articaine HCL and epinephrine in pharmaceutical
dosage form in the presence of excipients and additives in the
same concentration range as in the bulk without interference
with a recovery of 100.4 ± 0.65 and 99.2 ± 0.71, respectively. The
standard addition technique was further used to determine the
recovery of the proposed method (Table 5).
| Parameters |
Articaine HCl |
Epinephrine |
| Proposedmethod |
Reportedmethod [47] |
Proposedmethod |
Reportedmethod [47] |
| Linearity range (μg mL-1) |
0.32-8.34 |
20-70 |
0.32-8.34 |
1.5-4.0 |
| ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàN (number of replicates) |
9 |
6 |
9 |
6 |
| Mean % |
98.1 |
99.8 |
98.2 |
99.5 |
| SD* |
1.96 |
1.38 |
1.94 |
1.17 |
| Variance |
3.84 |
1.9 |
3.76 |
1.37 |
| t-test |
1.95
(2.77) |
|
1.61
(2.77) |
|
| F-test |
1.95
(6.39) |
|
2.74
(6.39) |
|
*Average of three determinations at each level; Figures in parenthesis are theoretical t and F values at 95% confidence level.
Table 5: Results obtained by the proposed electrochemical method compared with the reported method for the analysis of articaine HCl and epinephrine in Artinibsa®carpules.
Statistical analysis of the results obtained by the proposed
method compared with a reported method [47] revealed no
significant difference between the proposed and reported
methods, where the calculated t- and F- values are less than
the tabulated ones, confirming accuracy and precision at 95%
confidence limit [48]. However, the proposed method allows
simultaneous determination of both drugs, the reported method
determines both drugs separately (Table 6).
Concentration
(μmol L-1) |
%R
Articaine HCl |
%R
Epinephrine |
| 2 |
102.2 |
98.4 |
| 3 |
97.3 |
101.2 |
| 4 |
99.5 |
97.2 |
| 6 |
100.3 |
99.2 |
| 8 |
98.5 |
102.3 |
| 12 |
100.8 |
101.0 |
| 18 |
99.7 |
99.3 |
| 26 |
98.5 |
100.8 |
| Mean ±RSD |
99.6 ± 1.53 |
99.9 ± 1.68 |
Table 6:Simultaneous determination of articaine HCl and epinephrine in spiked urine samples.
The reported method involved determination of articaine HCl
and epinephrine by HPLC; articaine HCl was determined using
mobile phase (600 mL acetonitrile and 400 mL [1.3 mL from 1.0
mol L−1 sodium phosphate+32.5 mL from 0.5 mol L−1 disodium
phosphate in 1000 mL with distilled water (pH 8.0)], lichrospher
100 RP-18 (5 μm × 250 mm × 4 mm) as column and wavelength
of 240 nm. Epinephrine was determined using gradient mobile
phase [A: methanol, B: 0.42% w/v tetramethylammonium
hydrogen sulphate+0.116% w/v sodium heptanesulfonate+0.21%
v/v disodium edetate at pH 3.5 with 40% NaOH], Kromacil C18 5
μm × 4 mm × 150 mm and wavelength of 205 nm.
Simultaneous determination of articaine HCl and
epinephrine in spiked urine samples
Successive additions of articaine HCl and epinephrine solutions
(1.0 × 10−3 mol L−1) covering the linearity range of (1.0 × 10−6- 2.6
× 10−5 mol L−1) were added to the voltammetric cell containing
5 mL of diluted urine and 40 μL of 1.0 × 10−2mol L−1 SDS, the
voltammograms were recorded at a scan rate of 20 mV s−1 using
DPV at carbon paste electrode with percentage recoveries were
calculated from regression equation.
Conclusion
In the present work a simple, sensitive, accurate and precise
electroanalytical voltammetric method was developed for simultaneous determination of articaine HCl and epinephrine
in bulk, pharmaceutical dosage forms and spiked human urine
based on the electrochemical oxidation of these drugs at carbon
paste electrode in BR buffer of pH 7.0 in the presence of sodium
dodecyl sulphate, which allows to use the proposed method
for routine quality control applications for articaine HCl and
epinephrine.
References
- Budavari S (2002) The merck index: an encyclopedia of chemicals, drugs and biological. Merck and Co. Inc., White House Station, NJ, USA.
- Snoeck M (2012) Articaine: a review of its use for local and regional anesthesia. Local and Regional Anesthesia 5: 23-33.
- Whalen K, Finkel R, Panavelil TA (2015) Lippincott illustrated reviews: Pharmacology, Lippincott Williams and Wilkins. Philadelphia, New York, London.
- Deck DH (2012) Basic and clinical pharmacology. Mc Graw-Hill Medical, Singapore.
- Rustichelli C, Ferioli V, Gamberini G, Stancanelli R (2001) Enantiomeric separation of local anaesthetic drug by HPLC on chiral stationary phases. Chromatographia 54: 731-736.
- Richter K, Oertel R (1999) Solid-phase extraction and high-performance liquid chromatographic determination of articaine and its metabolite articainic acid in human serum. J Chromatogr B Biomed Sci Appl 724: 109-115.
- Hoizey G, Lamiable D, Gozalo C, Miric T, Thomas A, et al. (2009) Determination of articaine in human plasma by liquid chromatography-mass spectrometry and its application in a preliminary pharmacokinetic study. J Pharm Biomed Anal 49: 1082-1087.
- Schmidt M, Bracher F (2006) A convenient TLC method for the identification of local anesthetics. Pharmazie 61: 15-17.
- Manda CV, Popescu SM, Baniceru M (2015) Determination of articaine in human blood by gas and liquid chromatography and its application in a preliminary pharmacokinetic study. Inter J Drug Delivery Tech 5: 72-76.
- Kulkarni AP, Khan SKA, Zahee ZA, Dehghan MH (2011) Spectroscopic estimation of articaine hydrochloride. J Pharm Res 4:1596-1597.
- Mishra A, Upadhyaym A, Patra A, Chaudhury S, Chattopadhyay P (2009) Simultaneous determination of epinephrine and norepinephrine by high performance liquid chromatography. Sci Pharm 77: 367-374.
- Mishra KA, Mishra A, Chattopadhyay P (2010) A reversed-phase high performance liquid chromatographic method for determination of Epinephrine in pharmaceutical formulation. Arch App Sci Res 2: 251-256.
- Rane PV, Patil RK, Sangshetti NJ, Shinde BD (2008) Enantiomeric LC separation of epinephrine on amylose based sorbent. Chromatographia 67: 777-781.
- Carrera V, Sabater E, Vilanova E, Sogorb AM (2007) A simple and rapid HPLC-MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: Application to the secretion of bovine chromaffin cell cultures. J Chromatogr B 847: 88-94.
- Jebaraj AS, Prasanna R, Sivakumar T (2014) Analytical method development and validation of stability indicating HPLC method for estimation of fixed dosage form of atropine sulphate, epinephrine bitartarate and lignocaine hydrochloride injection. Int J Innov Pharm Sci Res 2: 1337-1348.
- Raja MMK, Patel RS, Mishra SH (2011) Identification and quantification of adrenaline from the leaves of clerodendrum phlomidis using thin-layer chromatography. J Chin Integr Med 9: 105-108.
- Alemany G, Akaarir M, Rossello C, Gamundi A (1996) Thin-layer chromatographic determination of catecholamines in rat plasma. Biomed Chromatogr 10: 225-227.
- Kudo M, Kudo T, Oyama T (1984) The simultaneous determination of plasma concentration of norepinephrine, epinephrine and dopamine by gas chromatography-mass spectrometry.The Jap J Anesthesiol 33: 1099-1103.
- Gyllenhaal O, Johansson L, Vessman J (1980) Gas chromatography of epinephrine and norepinephrine after derivatization with chloroformates in aqueous medium. J Chromatogr A 190: 347-357.
- Al-Ameri HAS (2016) Spectrophotometric determination of adrenaline in pharmaceutical preparations. Arab J Chem 9: 1000-1004.
- BulatovAV, Petrova AV, Vishnikin AB, Moskvin AL, Moskvin LN (2012) Stepwise injection spectrophotometric determination of epinephrine. Talanta 96: 62-67.
- Ekhtesasi A, Shishehbore MR (2016) Sensitive determination of epinephrine using kinetic spectrophotometric method. Orient J Chem 32: 467-472.
- El-Hawary, WF, Orabi RGH, Al-Yami SA (2015) Spectrophotometric determination of adrenaline via reaction with iodic acid. Int J Chem Sci 13: 563-575.
- Montaseri H, Khajehsharifi H, Yousefinejad S (2014) UV determination of epinephrine, uric acid and acetaminophen in pharmaceutical formulations and some human body fluids using multivariate calibration. Quimica Nova 37: 1404-1409.
- Lavanya N, Fazio E, Bonavita A, Leonardi GS, Neri G, et al. (2015) Simultaneous determination of epinephrine and uric acid in the presence of ascorbic acid using SnO2/graphene nanocomposite modified glassy carbon electrode. Sens Actuators B 221: 1412-1422.
- Taei M, Hadadzadeh H, Hasanpour F, Tavakkoli N, Dolatabadi MH (2015) Simultaneous electrochemical determination of ascorbic acid, epinephrine, and uric acid using a polymer film-modified electrode based on Au nanoparticles/poly(3,3',5,5'-tetrabromo-m-cresolsulfonphthalein). Ionics 21: 3267-3278.
- Goyal RN, Agrawal B (2012) Ag ion irradiated based sensor for the electrochemical determination of epinephrine and 5-hydroxytryptamine in human biological fluids. Anal Chim Acta 743: 33-40.
- Dorraji PS, Jalali F (2014) Novel sensitive electrochemical sensor for simultaneous determination of epinephrine and uric acid by using a nanocomposite of MWCNTsÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â chitosan and gold nanoparticles attached to thioglycolic acid. Sens Actuators B 200: 251-258.
- Li H, Wang X (2015) Simultaneous determination of epinephrine and uric acid at poly (guanine) modified glassy carbon electrode. Electrochemistry 83: 434-439.
- Moghaddam HM, Beitollahi H, Tajik S, Soltani H (2015) Fabrication of a nanostructure based electrochemical sensor for voltammetric determination of epinephrine, uric acid and folic acid. Electroanalysis 27: 2620-2628.
- El-ries MA, Mohamed GG, Attia AK (2008) Electrochemical determination of the antidiabetic drug repaglinide. J Pharma Soci Japan 128: 171-177.
- USP (2012) United States Pharmacopoeia, monographs Pharmacopoeial Forum, Asian Ed., Rand Mc Nally, USA.
- Attia AK (2009) Determination of antihypertensive drug moexipril hydrochloride based on the enhancement effect of sodium dodecylsulfate at carbon paste electrode. Talanta 81: 25-29.
- Shankar SS, Swamy BEK (2014) Detection of epinephrine in presence of serotonin and ascorbic acid by TTAB modified carbon paste electrode: a voltammetric study. Int J Electrochem Sci 9: 1321-1339.
- David IG, Popa DE, Caklin A, Buleandra M, Iorgulescu E (2016) Voltammetric determination of famotidine on a disposable pencil graphite electrode. Turk J Chem 40: 125- 135.
- Vittal R, Gomathi H, Kim K (2006) Beneficial role of surfactants in electrochemistry and in the modification of electrodes. Advan Colloid Interface Sci 119: 55-68.
- Niranjana E, Swamy BEK, Naik RR, Sherigara BS, Jayadevappa H (2009) Electrochemical investigations of potassium ferricyanide and dopamine by sodium dodecylsulphate modified carbon paste electrode: a cyclic voltammetric study. J Electroanal Chem 631: 1-9.
- Zhang S, Wu K, Hu S (2002) Voltammetric determination of diethylstilbestrol at carbon paste electrode using cetylpyridine bromide as medium. Talanta 58: 747-754.
- Shankar S, Swamy K, Chandra U, Manjunatha JG, Sherigara BS (2009) Simultaneous determination of dopamine, uric acid and ascorbic acid with CTAB modified carbon paste electrode. Int J Electrochem Sci 4: 592-601.
- Cao X, Xu Y, Luo L, Ding Y, Zhang Y (2010) Simultaneous determination of uric acid and ascorbic acid at the film of chitosan incorporating cetylpyridine bromide modified glassy carbon electrode. J Solid State Electrochem 14: 829-834.
- Hu C, He Q, Li Q, Hu S (2004) Enhanced reduction and determination of trace thyroxine at carbon paste electrode in the presence of trace cetyl trimethyl ammonium bromide. Anal Sci 20: 1049-1054.
- Jain R, Dwivedi A, Misha R (2008) Voltammetric behavior of cefdinir in solubilized system. J Colloid Interface Sci 318: 296-301.
- Jain R, Rather VJA (2011) Voltammetric behaviour of drotaverine hydrochloride in surfactant media and its enhancement determination in Tween 20. Colloids and Surfaces B: Biointerfaces 82: 333-339.
- Abbar JC, Nandibewoor ST (2013) Voltammetric oxidation and determination of atorvastatin based on the enhancement effect of cetyltrimethyl ammonium bromide at a carbon paste electrode. Colloids and Surfaces B: Biointerfaces 106: 158-164.
- Gosser DK (1993) Cyclic voltammetry: Simulation and analysis of reaction mechanism, VCH, NewYork.
- ICH (2005) Validation of analytical procedures: Text and methodology Q2. Proceeding of the International Conference on Harmonisation of Technical Requirement for Registration of Pharmaceuticals for Human Use, London, England.
- HPLC method adopted by Alexandria Company, Alexandria, Egypt.
- Mendham J, Denny RC (2008) Vogels Textbook of quantitative chemical analysis. Barnes and Thomas, London, England.