Keywords
Sleep disorders; Systemic Lupus Erythematosis (SLE); Children
Introduction
About one fifth of SLE patients were diagnosed in pediatric age known as Juveil onset SLE (jSLE). 1-5 jSLE had a more worse outcome than in adulthood, with more disease damage, more frequency of lupus nephritis (LN), blood diseases, photo hypersensitivity, dermatological or mucosal involvement. jSLE had higher morbidity and mortality rates [1].
Disturbances of sleep are commonly encountered but poorly documented in adult patients with SLE [2].
A higher prevalence of sleep disturbances has been reported in other rheumatic diseases, such as rheumatoid arthritis and Sjogren’s syndrome [3-6].
The impacts of sleep abnormalities on quality of life may be attributed either to disruption of sleep, difficulties in start of sleep onset or difficulties in maintaining sleep. There are few publications concerning sleep disorders in SLE. McKinley et al., who assessed sleep quantity and quality through a nonstandardized sleep questionnaire, found that the sleep disruption reported by the SLE patients had a significant and strong effect on fatigue level and that the effects of disease activity on fatigue were mediated primarily by sleep disruption rather than by depressive mood of patients [7].
The aim of this work was to assess objective and subjective evidences of sleep disorders in jSLE and to detect whether there was relation between active lupus nephritis and sleep disturbances in pediatric patients.
Materials and Methods
Study design
This study was prospective case-control study carried out in the period from August 2016 to August 2017 after approval from the research ethical committee of the faculties of Medicine of Tanta University and informed written or verbal consents from parents of included patients on 50 Lupus patients with ages ranged from 10-18 years who were selected from outpatient clinic and inpatient wards of Pediatric Nephrology Unit of Pediatric Department of Tanta University Hospital who fulfilled the revised criteria of American College of Rheumatology (ACR) for diagnosis of SLE [8,9].
They were subdivided into 2 groups, group 1 included 50 known cases of SLE during renal disease activity (Active lupus nephritis) and group 2 included 30 known cases of SLE without activity (inactive SLE).
Disease activity was evaluated according to the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score which is a validated disease activity measure for childhoodonset SLE with a total score of 0-105. The tool consists of 24 weighted items grouped into the following nine domains: Central Nervous system (CNS), vascular, renal, musculoskeletal, espousal, dermal, immunological, constitutional and hematological diseases [10-13]. Disease onset occurred 6-120 months prior to this study.
Inclusion criteria
All children and adolescents of both sexes with SLE fulfilled the revised criteria of ACR for diagnosis after 6 months of disease onset.
Exclusion criteria
Children less than 10 years of age, as they could not reliably comprehend the sleep quality assessment questionnaire.
Experimental Procedure
All patients were subjected to
1. Taking full history: Included age, sex, history of CNS diseases or developmental disorders.
2. Thorough clinical examination: Included anthropometric measurements for assessment of nutritional status which included weight, height, Body mass index (BMI), Mid Arm Circumference (MAC) and vital signs especially arterial blood pressure which was measured by auscultatory method using a mercury sphygmomanometer, in the semi-sitting position after 10 minutes of rest, using an appropriate sized cuff and was taken as the mean value of 3 successive readings in 3 different days.
3. Routine laboratory investigations: Included Complete blood count (CBC), Erythrocyte sedimentation rate (ESR), complete urine analysis for 24 hr. urinary proteins, BUN, serum creatine, serum complements (C3 and C4) levels and Anti-ds DNA and antinuclear antibody (ANA) which were performed after morning awakening on the first day of the study.
4. Renal biopsy: The renal biopsy was done retrospectively for all cases to determine the severity of Lupus Nephritis (LN). The pathologic changes present on kidney biopsy help guide treatment decisions and may be predictive of long-term kidney survival; the histological findings are graded using the International Society of Nephrology/ Renal Pathology Society (ISN/RPS) Classification. Class I and II used for purely mesangial involvement (I, mesangial immune deposits without mesangial hypercellularity; II, mesangial immune deposits with mesangial hypercellularity), class III for focal glomerulonephritis (involving<50% of total number of glomeruli) with subdivisions for active and sclerotic lesions; class IV for diffuse glomerulonephritis (involving>or=50% of total number of glomeruli) either with segmental (class IV-S) or global (class IV-G) involvement, and also with subdivisions for active and sclerotic lesions; class V for membranous lupus nephritis; and class VI for advanced sclerosing lesions [14-16].
5. Validated pediatric sleep quality assessment questionnaire: This was a subjective method to evaluate quality of sleep by taking detailed history about complaints of children or adolescents during sleep. It fulfilled the following items: Onset of delayed sleep, awakening during night time, difficulty to get back to sleep after awakening, difficult arousal in the morning, sleepiness during day time, pain at limbs and snoring. Sleep room characters were also studied [17].
6. The polysomnographic studies (Polysomnography, PSG): Overnight PSG sleep recording was performed by well trained and experienced staff for all patients at the Sleep Laboratory of the Neuropsychiatry Department of TUH. It included the measurements 12-lead electrocardiogram (ECG), pulse oximetry of electro-oculography (EOG) of right and left ocular movements (ROC, LOC), 4 channel electroencephalogram (EEG) (C3, C4, 01, 02), electromyogram at chin and leg surfaces, chest movements, oronasal airflow, snoring, and body position channel [18].
Scoring of PSG
Blindly to the demographic and clinical characteristics of the studied children, Data was completed, processed and scored following identical procedures. The American Sleep Disorders Association criteria defined arousals by noticing three seconds of wake EEG. which was defined as at least one 30-second epoch of wakefulness scored after sleep onset [19]. Respiratory movements were monitored using piezoelectric bands for thoracic and abdominal effort. Complete cessation of respiration more than ten seconds was identified as central apnea while obstructive apnea was cessation of oronasal airflow was monitored by a 4-lead thermistor system for at least 10 seconds, despite persistent respiratory effort. Hypopnea was defined as reduction of respiratory airflow from a third to a half of normal value for age. 18 Sleep disordered breathing (SDB) was diagnosed if the respiratory disturbance index (RD I) which was the total number of apnea and hypopnea per hour of sleep was more than five per hour of sleep. 18 Periodic limb movements (PLMs) were scored according to Coleman’s criteria. 20 A movement was scored when it occurred as part of a series of 4 consecutive movements that were separated by at least 4, but not more than 90, seconds with a duration of 0.5–5 seconds. PLMs disorders (PLMD) were diagnosed if the PLM index (PLMI) exceeded five per hour of sleep.18 Quantitative evaluation of sleep stages was made visually according to conventional Rechtschaffen and Kales 21 criteria with 30 second epochs for rapid eye movement (REM) and non-REM sleep. Oxygen saturation (SaO2) was recorded with a pulse oximeter (BCI, Waukesha, WI). All recordings were made at an emulated paper speed of 10 mm/second. Correlations between the subjective (pediatric sleep quality assessment questionnaire) and the objective findings (PSG) were statistically analysed. Also, correlation between sleep disorders and disease activity was calculated.
Statistical Analysis
The statistical significance between the studied groups as regard demographic, laboratory and PSG data were expressed as mean ± SD, and were tested using a 2-sample student t-test. The statistical significance between the group studied as regard sex distribution and subjective data obtained by the sleep quality questionnaire were expressed in number of patients and percentage and were tested using the chi square test (X2 test). P value was considered significant if<0.05.
Results
Demographic data of the studied patients were summarized in Table 1. The age in studied active LN patients ranged between (10-18) years with a mean of 13.8 ± 2.6 while in group 2, age ranged between (10-17) years with a mean of 13 ± 2.
| Variables |
Group (1)
Active lupus nephritis |
Group (2)
Inactive SLE |
P-value |
| Age (years) |
Range |
10-18 |
10-17 |
>0.05 |
| Mean ± SD |
13.8 ± 2.6 |
13 ± 2 |
| Sex |
Male: No (%) |
3 (6%) |
4 (13.3%) |
>0.05 |
| Female: No (%) |
47 (94%) |
26 (86.7%) |
| Duration (Month) |
Range |
12-120 |
6-72 |
0.417 |
| Mean ± SD |
42.4 ± 38.2 |
33.2 ± 20.4 |
| SLEDAI |
Range |
8-25 |
2-4 |
<0.001* |
| Mean ± SD |
13.4 ± 4.9 |
3 ± 0.93 |
| Clinical and serological manifestations of SLE in studied patients |
| Haematological manifestations |
No (%) |
33 (66%) |
0 (0%) |
<0.001* |
| Renal manifestations |
No (%) |
33 (66%) |
8 (26.67%) |
0.003* |
| Musculoskeletal manifestations |
No (%) |
37 (74%) |
6 (20%) |
0.003* |
| Skin/MM manifestations |
No (%) |
40 (80%) |
6 (20%) |
0.003* |
| Constitutional manifestations |
No (%) |
47 (94%) |
6 (20%) |
0.004* |
| CNS manifestations |
No (%) |
7 (14%) |
0 (0%) |
0.334 |
| Histopathological classes of studied patients by renal biopsy classes |
Lupus nephritis class I: No (%) |
25 (50%) |
- |
- |
| Lupus nephritis class II: No (%) |
5 (10%) |
- |
- |
| Lupus nephritis class III: No (%) |
15 (30%) |
- |
- |
| Lupus nephritis class IV: No (%) |
2 (8%) |
- |
- |
| Lupus nephritis class V: No (%) |
3 (6%) |
- |
- |
| Anti-ds DNA Antibody (U/ml) |
Range |
10-580 |
10-325 |
0.013* |
| Median |
10-580 |
100 |
Mann-Whitney Test as used for comparing between median of Anti-ds DNA of the studied groups.
Table 1: Demographic, histopathological and laboratory data of studied groups.
Among 50 patients with active lupus nephritis, 3 (6%) were males and 47 (94%) were females and among 30 patients with inactive SLE, 4 (13.3%) were males and 26 (86.7%) were females with Female: Male ratio 15.2:1 and 6.5:1 in groups 1 and 2 respectively.
There was insignificant difference between studied patients and controls as regard age and sex as shown in Table 1.
The mean of disease duration was 42.4 ± 38.18 in LN group and 33.2 ± 20.4 in inactive group. There was significant difference between patient’s groups regarding their SLEDAI score. Mean value of SLEDAI score in SLE patients with active LN was 13.4 + 4.9 and ranged from 8-25 while in inactive SLE patients, its mean was 3 + 0.93 and ranged from 2-4 (p<0.05) as shown in Table 1.
All clinical manifestations of SLE were significantly higher in active LN patients than in inactive SLE patients except for CNS manifestations which were present only in 7 (14%)of active patients but didn't show statistically significant difference as shown in Table 1.
Group 1 patients had significantly higher levels of serum Anti-ds DNA.
Renal biopsy in SLE children patients with active LN according to the International Society of Nephrology (ISN) lupus nephritis grading system. were shown in Table 2, ISN Classes (50%) and III (30%) were the commonest findings.
| Variables |
Active LN patients (No=50) |
Inactive SLE patients (No=30) |
P-value |
| % |
No. |
% |
No. |
| Awakening during night |
38 |
76 |
8 |
26.7 |
0.002* |
| Snoring |
8 |
16 |
2 |
6.7 |
0.744 |
| Sharing of sleeping room |
36 |
72 |
10 |
33.3 |
0.017* |
| Hypnotic medication |
22 |
44 |
0 |
0 |
0.003* |
| Restless legs (a feeling of crawling, aching or inability to keep legs still). |
11 |
22 |
3 |
10 |
0.03* |
| Difficult arousal at morning and excessive sleepiness during day time |
21 |
42 |
3 |
10 |
0.001* |
| Naps during day times |
30 |
60 |
18 |
60 |
0.388 |
Table 2: Subjective sleep data of cases and control subjects.
As regard medications received for our studied patients: 31 (38.75%) of patients were receiving corticosteroids plus azathioprine, 23 (28.75%) were receiving steroids plus azathioprine plus chloroquine, 17 (21,25%) were receiving chloroquine plus non-steroidal anti-inflammatory drugs (NSAID), 6 (7.5%)was receiving only antihypertensive medication, and 3 (3.75%) received no medication as noncompliance to follow up treatment.
Table 2 summarized differences in subjective data obtained by the sleep questionnaire in the studied groups. The active LN patients reported poorer sleep at night, more frequent awakenings, more restlessness, greater disturbed sleep, and more problems with sleepiness and fatigue than the control subjects (Table 3).
| Variable |
Active Lupus Nephritis group (n=50) |
Inactive Lupus group (n=30) |
Statistical test |
| Mean |
SD |
Mean |
SD |
t |
P |
| Sleep efficacy % |
85.8 |
8.6 |
92.2 |
3.9 |
2.2 |
0.005* |
| Sleep latency (min) |
40.16 |
15.39 |
39.06 |
10.38 |
0.243 |
0.809 |
| Arousal index |
2.67 |
1.66 |
1.62 |
1.21 |
1.425 |
0.021* |
| SWS % |
12.22 |
6.31 |
19.13 |
3.68 |
1.725 |
0.043* |
| RDI |
8.84 |
8.46 |
1.89 |
2.09 |
2.96, |
<0.02* |
| PLMI |
8.25 |
5.38 |
3.17 |
2.29 |
3.19 |
<0.006* |
| REM % |
9.09 |
4.84 |
8.6 |
3.86 |
0.331 |
0.742 |
| NREM % |
90.87 |
4.84 |
92.06 |
3.91 |
0.805 |
0.426 |
| SaO2 Min |
82.05 |
7.29 |
89.44 |
3.38 |
5 3.36 |
<0.004*. |
| Highest heart rate |
133.20 |
17.29 |
135.4 |
13.73 |
-0.419 |
0.678 |
| Lowest heart rate |
54.84 |
10.36 |
59.66 |
16.24 |
-1.15 |
0.257 |
SLE: Systemic lupus erythematosus; RDI: Respiratory disturbance index, SaO2 Min: Minimum oxygen saturation; PLMI: Index of periodic limb movement
Table 3: Polysomnography data in the hemodialysis and the control groups.
There was significant increase in prevalence of day time symptoms in the active LN children when compared to group 2, which included difficult morning arousals and excessive day time sleepiness and limb pains. Also, there was a significant increase in prevalence of night time symptoms in active LN children when compared to healthy controls including night time awakening and room sharing during night.
No significant differences between 2 studied groups as regard the prevalence of snoring and day time naps. In addition, there was also a significantly higher need for hypnotic medications in the active LN group when compared to the inactive group.
Table 3 compared between the studied patients and controls as regard the overnight PSG measurements as objective parameters. There was significant increase in values of sleep efficacy (%) and arousal index in group 1 than in group 2 (P<0.05).
There was a significant decrease in slow wave sleep (SWS) value in the group 1when compared to group 2 (P=0.043).
There was significant increase in respiratory disturbance index (RDI), PLMD and minimum oxygen saturation (SaO2 Min % in active LN group than in inactive group (P<0.05).
There was no significant difference between the active LN patients and inactive SLE group as regard sleep latency, rapid eye movement sleep (REM), non-rapid eye movement sleep (NREM) and range of heart rate (the highest and the lowest value) (p>0.05).
As regard correlation between subjective and objective methods of sleep assessment, there was a significant positive correlation between ‘restless legs’ as a feeling of crawling, aching, inability to keep legs still and the PLMI (r=0.593 and P=0.05) (Figure 1).
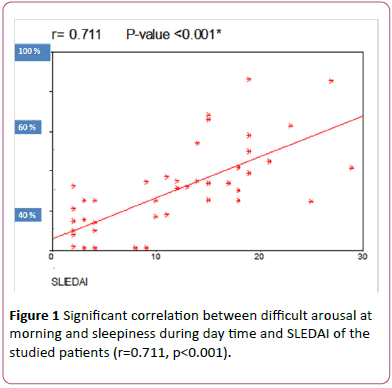
Figure 1: Significant correlation between difficult arousal at morning and sleepiness during day time and SLEDAI of the studied patients (r=0.711, p<0.001).
Discussion
The overall quality of life of children and adolescents with SLE was markedly affected by the disturbances of sleep patterns.
There are few publications which had evaluated architecture of sleep in pediatric patients with SLE using subjective or objective methods e.g. PSG.
Our Pediatric Nephrology Units in the 3 involved Universities are highly specified in managing of lupus nephritis so children and adolescents were recruited from different locations region including Gharbia, Fayoum, Sharkia, Kafr El-Sheik, Menoufia and Behira Governates. Thus, our data represents different areas of middle Egypt.
This work aimed to report subjective evidences of disordered sleep patterns in children and adolescents with SLE using pediatric sleep quality questionnaire as compared to the control group.
This was confirmed by objective evidences of disturbed sleep architecture noticed in the SLE group on analysis of data of PSG.
Commonly reported sleep disorders as subjective complains in lupus patients included restless leg syndrome (RLS), difficulty in arousal at the morning, night awakening, prolonged day time sleepiness and sleep disordered breathing (SDB). Whether the apparently high prevalence of sleep disorders in lupus patients may be contributed to the symptom of fatigue which was common in SLE population in general.
Previous publications which objectively assessed sleep architecture in patients with SLE by using polysomnography (PSG) had reported shorter sleep times, less slow wave sleep (SWS%), fragmented sleep and periodic limb movement disorders (PLMD).
Sleep disorders may result in deficient sleep, bad-quality sleep or day time malfunction, in form of excessive day time sleepiness, emotional disorders, decreased work performance and increased liability to trauma such as road traffic accidents and falling from height [19-23].
In our study, the SLE patients were sleepier during the day than the healthy control group and reported having problems due to the sleepiness and fatigue.
Because the use of steroids can induce changes in sleep, a separate analysis of sleep disorders among patients taking corticosteroids and those who were not was done. That analysis disclosed no differences between patients who were taking prednisone and those who were not [24]. These findings were similar to those in the study by Mahowald et al., who found identical sleep patterns in rheumatoid arthritis patients who were receiving corticosteroids and those who were not [25].
In the present study, symptoms of sleep disturbances were more frequent in Active LN group when compared with Inactive SLE patients.
The high prevalence of sleep disturbances in our study is similar to that reported in previous studies among adults with SLE.
In this study, there was statistically significant increase in behavioural sleep problems (awakenings during night, sharing of sleeping room and using hypnotic medication) among children with active lupus nephritis as compared to other groups (P=0.002, 0.017 and 0.03 respectively).
According to subjective analysis of sleep architecture, the results of this study showed, that 8 (16%) had a snoring in LN group while only two (6.7%) had a snoring in inactive group.
In this work, Restless legs symptoms were detected in 11 (22%) of the studied children with Active LN group and only in 3 (10%) of inactive SLE group. A primary sleep disorder such as restless legs syndrome may be the presenting feature of SLE.
The paediatrician should be aware that a complain of arthralgia might be attributed to the SLE disease itself or the presence of a concomitant rheumatological disease such as fibromyalgia. The latter had from one quarter to a half comorbidity incidence of occurrence with SLE patients as mixed connective tissue disease [26].
We reported excessive sleepiness during day time in 21 (42%) of the studied children with active LN versus only 3 (10%) of inactive SLE group.
Our findings are consistent with those of previous studies among children with CKD [27-29].
PSG as a diagnostic tool for sleep patterns in children was more informative than sleep questionnaire especially for PLMD, RDI and sleep staging. This study documented objectively assessed, polysomnographically measured sleep disorders and reported sleep disturbances in patients having active LN without secondary concomitant fibromyalgia (which was a common association in previous adult studies).
The results of this study showed that there was a statistically significant increase in mean value of RDI in active LN group when compared to inactive group (8.84 + 8.46 and 1.89 + 2.09 respectively) (p<0.02). This is in accordance with Valencia-Flores M, et al. [10] who reported comparable results. In our study, there was significant increase in patients presented with some breathing alterations as evidenced by lower SaO2 (with statistically significant difference) (P<0.004) and lower figure of both highest and lowest heart rate during sleep (but without with statistically significant difference) (P=0.678 and 0.257 respectively) in LN group when compared to inactive group.
In our study, there was significant increase in the objective criteria for movement disorder during sleep evidenced by higher mean of PLMI in the studied patients with active LN when compared to inactive lupus patients (p<0.006). This finding increased the complexity of evaluating the complaint of leg pain in patients with SLE.
In previous studies, it was known that patients with obstructive sleep apnea may complain of restlessness and had agitated behaviour with gross movements which ranged from simple movements of the extremities, including typical periodic leg movements to greater movements of the arms and legs that cause unwitting slaps or kicks to the bed partner. To this extent, it is possible that in some of the patients, the movement alterations might be related to alterations in respiration. Treatment of breathing disorders could rule out this possibility.
Different mechanism was postulated to explain the presence of primary sleep disorders in patients with initial stages of CKD as active LN. They included psychosocial factors, low production of melatonin and circadian rhythm disruption [30].
As regard correlation between subjective and objective methods of sleep assessment, there was a significant positive correlation between ‘restless legs’ as a feeling of crawling, aching, inability to keep legs still and the PLMI (Spearman’s correlation coefficient was 0.593 and P=0.05). So, it was recommended to inquire about sleep disturbances by “restless legs” in lupus patients. Also, there was significant positive correlation between difficult arousal at morning and sleepiness during day time and SLEDAI of the studied patients. (r=0.711, p<0.001) which indicate strong relation between sleep patterns and disease activity.
Conclusion
There is high incidence of sleep disorders in pediatric patients with SLE. The most frequent were respiratory presented with some breathing alterations and movement disorders e.g. PLMD.
Disclosure
The authors declare no conflicts of interest.
References
- Mahajan A, Herrmann M, Munoz LE (2016) Clearance deficiency and cell death pathways: A model for the pathogenesis of SLE. Front Immunol 7: 35.
- Ramos PS, Brown EE, Kimberly RP, Langefeld CD (2010) Genetic factors predisposing to systemic lupus erythematosus and lupus nephritis. Semin Nephrol 30: 164-176.
- Ohl K, Tenbrock K (2011) Inflammatory cytokines in systemic lupus erythematosus. Biomed Res Int 3: 15.
- Zhu J, Wu F, Huang X (2013) Age-related differences in the clinical characteristics of systemic lupus erythematosus in children. Rheumatol Int 33: 111-115.
- Concannon A, Rudge S, Yan J, Reed P (2013) The incidence, diagnostic clinical manifestations and severity of juvenile systemic lupus erythematosus in New Zealand Maori and Pacific Island children: The Starship experience (2000-2010). Lupus 22: 1156-1161.
- Malattia C, Martini A (2013) Paediatric-onset systemic lupus erythematosus. Best Practic Res Clin Rheumatol 27: 351-362.
- Morgan TA, Watson L, McCann LJ, Beresford MW (2013) Children and adolescents with SLE: Not just little adults. Lupus 22: 1309-1319.
- Gormezano NW, Silva CA, Aikawa NE, Barros DL, Da Silva MA, et al. (2016) Chronic arthritis in systemic lupus erythematosus: Distinct features in 336 paediatric and 1830 adult patients. Clin Rheumatol 35: 227-231.
- Choi JH, Park DJ, Kang JH, Yim YR, Lee KE, et al. (2015) Comparison of clinical and serological differences among juvenile, adults, and late-onset systemic lupus erythematosus in Korean patients. Lupus 24: 1342-1349.
- Valencia-Flores M, Resendiz M, Castan VA, Santiago V, Campos RM, et al. (1999) Objective and subjective sleep disturbances in patients with systemic lupus erythematosus. Arthritis Rheum 42: 2189-2193.
- Drewes AM, Svendsen L, Taagholt SJ, Bjerregard K, Nielsen KD, et al. (1998) Sleep in rheumatoid arthritis: A comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol 37: 71–81.
- Gudbjornsson B, Broman JE, Hetta J, Halogren R (1993) Sleep disturbances in patients with Sjogren’s syndrome. Br J Rheumatol 32: 1072-1076.
- McKinley PS, Ouellette SC, Winkel GH (1995) The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus: A proposed model. Arthritis Rheum 38: 826–834.
- Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725-1734.
- Bertsias GK, Ioannidis JP, Aringer M, Bollen E, Bombardieri S, et al. (2017) EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: Report of a task of the EULAR standing committee for clinical affairs. Ann Rheum Dis 69: 2074-2823.
- Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, et al. (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241-250.
- Chervin RD, Hedger KM, Dillon JE, Pituch KJ (2000) Pediatric Sleep Questionnaire (PSQ):Validity and reliability of scales for sleep disordered breathing, snoring sleepiness and behavioral problems. Sleep Med 1: 21-32.
- Judi A, Judith A (2003) A clinical guide to pediatric sleep. Diagnosis and management of sleep disorders. Lippincott Williams, Philadelphia, USA. 13: 106-121.
- Report AS (1992) EEG Arousals: Scoring rules and examples: A preliminary report from the sleep disordered Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 173-84.
- Koo B, Bagai K, Walters A (2016) Restless legs syndrome: Current concepts about disease pathophysiology. Tremor Other Hyperkinet Mov (N Y) 6: 401.
- Rechtschaffen A, Kales A (1986) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. US Department of Health, Education, and Welfare; National Institutes of Health 204.
- Khothari CR (2012) Research methodology, methods and Techniques, New Age International, In: Khothari CR. New Delhi, India.2: 95-97.
- Paker KP, Kuntner NG, Bliwise DL, Baiely DL, Rye DB (2003) Nocturnal Sleep, day time sleepiness, and quality of life in stable patients on hemodialysis. Health Qual Life Outcomes 1: 68.
- Sinha R, Davis ID, Matsuda-Abedini M (2009) Sleep disturbances in children and adolescents with none dialysis-dependent chronic kidney disease. Arch Pediatr Adolesc Med 163: 850e5.
- Mahowald MW, Mahowald ML, Bundlie SR, Ytterberg SR (1989) Sleep fragmentation in rheumatoid arthritis. Arthritis Rheum 32: 974-983.
- Middleton GD, McFarlin JE, Lipsky PE (1994) The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 37: 1181–1188.
- Riar SK, Leu RM, Turner-Green TC, Rye DB, Kendrick-Allwood SR, et al. (2013) Restless legs syndrome in children with chronic kidney disease. Pediatr Nephrol 28: 773e95.
- Applebee GA, Guillot AP, Schuman CC, Teddy S, Attarian HP (2009) Restless legs syndrome in pediatric patients with chronic kidney disease. Pediatr Nephrol Berl Ger 24: 545e8.
- Davis ID, Greenbaum LA, Gipson D, Wu LL, Sinha R, et al. (2012) Prevalence of sleep disturbances in children and adolescents with chronic kidney disease. Pediatr Nephrol 2012;27:451e9.
- De Santo RM, Bartiromo M, Cesare CM, Cirillo M (2008) Sleep disorders occur very early in chronic kidney disease. J Nephrol 21: S59e65.

