Research Article - (2022) Volume 7, Issue 1
Received: 03-Dec-2021 Published: 23-Dec-2021
Zeolite nanoparticles are synthesized by hydrothermal method and Zeolite polypyrrole polymer nanocomposite is chemically polymerized. The structural, morphological, and chemical features of the Zeolite LTL/PPy material synthesized were investigated. The crystalline character of the materials may be seen in the powder XRD. The uniform hexagonal rod structure of the zeolite can be seen in SEM micrographs. In the case of the zeolite polypyrrole, SEM micrograph polypyrrole is encapsulating the zeolite; it is also observed that polypyrrole spherical balls are formed. The gas sensing substance is a polymer nanocomposite called Zeolite polypyrrole. The sensor can detect ammonia at a concentration of 1 ppm in less than a minute, with a response and recovery times of 15 and 35 seconds, respectively. The sensor has a sensitivity of 12.34 ppm-1. The polymer composite can degrade the methylene blue dye in the presence of UV light up to 79.4 %.
Zeolite; Nanoparticle; Synthesis; Sensing
Nanotechnology is known as a field of research and innovation which is concerned since the last century. Nanoparticles are materials with particle sizes ranging from 1 to 100 nanometers [1]. Nanoparticles are employed in a wide range of applications, including nanomedicine [2], optoelectronics [3], and chemical sensors [4]. Zeolites are crystalline microporous aluminosilicates with tetrahedral structures made up of corner-sharing AlO4 and SiO4. Zeolite is a microporous substance with a homogeneous range of pore opening sizes. The term “micro-porous” can also be represented as “nanoparticle” to indicate the nanometer-scale [5]. Zeolites are widely used in various applications such as water softeners [6], adsorbents [7] and industrial catalysis reactions [8], gas sensing [9]. Zeolites are also employed in the pyrolysis method of plastic breakdown. The zeolite nanostructures have many advanced properties like large internal surface area, unique channel systems, high pore volume, and adjustable active sites [10]. Zeolite nanoparticles are becoming more appealing for synthesis because of their high crystallinity, more action rides, and increased surface areas [11]. Zeolite shows many promising properties such as absorbent, ion exchange, toxic chemical, and catalyst for the synthesis of various chemicals. The ion exchange process is the most important property of zeolite. Catalytic and adsorption properties are reported. In the field of gas detection, zeolite has a lot of potentials. The use of zeolite as a gas sensing material has been described by several researchers. It contains a large number of recent experimental researches. Zeolites, as a host material, tend to boost the reactivity of composite other materials to a specific gas and provide active sites for the target gas's adsorption. Due to their unique qualities like high surface area, high thermal and chemical stability, and outstanding adsorption properties, presence in mobile ions, and hydrophobic or hydrophilic properties, zeolite-based materials or composites have attracted a lot of attention for use in gas sensing applications [12-14]. Zeolite L, also known as LTL (Linde Type L), has a large pore size and 12 membered ring zeolites with a 1-D channel system. It is discovered in 1968 by Breck and Flaningen as synthetic material and observed many years later like the zeolite structure by Artioli and Kvick [15]. With a=1.84 nm and c=0.75 nm, Zeolite L has a hexagonal symmetry unit cell. These have a 1-D massive pore system that runs parallel to the crystal structure's c-axis [16]. Polyaniline (PANI), polypyrrole (PPy), poly (thiophene) (PTh), and Poly (P-Phonoylene) (PPP) are examples of conducting polymers with gas sensing abilities [17-20]. There are found many uses in sensing fields of a polymer such as biosensors, humidity sensors, ion selectivity sensors, pH sensors, and gas sensors [21-25]. Because of its unique properties such as good electrical conductivity, easy synthesis, thermal and high environmental stability, low energy band-gap, oxidation state, counter ions or dopants, and low-cost processing, conducting polypyrrole has been identified as one of the most promising conductive polymers [26]. Both electrochemical and chemical processes can be used to make conducting Polypyrrole (PPy) gas sensing materials. Polypyrrole is widely used in various fields such as microbial fuel cells, absorbing materials, super-capacitors, gas sensing [27-30].
In this present work, we are reporting the synthesis of zeolite LTL and its composite with conducting polypyrrole. SEM-EDX, FTIR, and XRD are used to characterize the zeolite and PPy/Zeolite polymer nanocomposite. The polymer nanocomposite is utilized to detect harmful ammonia gas as a sensing material.
Materials
Potassium hydroxide, silica sol, aluminum hydroxide, magnesium nitrate, silver nitrate, pyrrole is purchased from fisher scientific, sigma, CDH company, Merck, fisher scientific, sigma, respectively. The entire chemical utilized in the synthesis is analytical grade, therefore no additional purification is required.
Synthesis of zeolite LTL
The synthesis of zeolite L was carried out by dissolving 10.13 g potassium hydroxide and 5.27 g aluminum hydroxide in 16.66 ml double-distilled water, then heating the reaction mixture until a clear solution 'A' was formed. The temperature of Solution A is then lowered to room temperature, and the water loss due to heating is compensated for. 4.83 g magnesium nitrate solution was added to 33.0 ml water in a separate beaker 50.08 ml silica sol and combined by stirring for about 3 minutes until a homogenous solution 'B' was achieved. Then, in a vicious mature form, solutions A and B are mixed, and 8.33 ml of water is added to them. The solution is agitated until it begins to thicken. The gel is placed in a Teflon-lined autoclave and held at 175 degrees Celsius for 48 hours. The autoclave is removed from the oven and immersed in cool water after 48 hours. The material inside the autoclave is centrifuged at 10,000 rpm, rinsed until pH is 9, and then dried in an oven for 16 hours at 1500C. The dry material is ground into a fine powder and calcined for 7 hours at 540 degrees Celsius [31].
Modified zeolite L
The ion exchange process is used to modify Zeolite L. 1 g zeolite and 0.2548 g Ag (NO)3 were mixed in 50 ml double distilled water and left in the darkroom for 4 hours at 50 oC. The resulting solution was filtered and washed with double deionized water before being dried at 110 oC for 16 hours. As a result, this product was calcined at 550°C for 4 hours.
Composite of Zeolite/PPy
After dissolving 1 g Zeolite Ag-LTL powder and 0.5 ml pyrrole in 35 ml deionized water and sonicating for 15 minutes, 0.36 M FeCl3.6H2O (35 ml water) is added and agitated for 2 hours. Following this, 20 mL acetone was added to the aforementioned solution to terminate the polymerization reaction. Finally, the composite filters were rinsed in double deionized water and dried in an oven at 50 degrees Celsius for 24 hours [32].
Characterization
X-Ray Diffraction (XRD) tests utilising a Rigaku Miniflex 600 diffractometer at 40 kV and 30 mA in the 2o to 90o range with CuK (0.15406) radiation are used to investigate the structural properties of the synthesised material. The Philips Model-Quanta 200 FEG was utilised to undertake morphological and compositional analysis utilising Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS). A PerkinElmer spectrometer was used to record the FT-IR spectrum, which ranged from 450 to 4000 cm-1.
Gas sensing Measurement
As indicated in Fig. 1, gas sensing is carried out in a 1 L handmade glass chamber. The sensing material is drop cast between the two copper electrodes and connected to the multimeter. The change in resistance in the sensing material when the ammonia gas is introduced is recorded on the multimeter. Inside the gas chamber, different concentrations of ammonia gas are introduced. After every exposure of ammonia gas, recovery of the sensing material is carious out by passing air inside the chamber. The required ammonia gas concentration is estimated using the equation published in the literature [33].
C = × 1000
Where C is the desired target gas concentration in parts per million (ppm), q is the density of the liquid (gas) in grams per milliliter (g/mL), V' is the volume of the liquid (gas), T is the temperature in Kelvin, M is the molecular weight of the liquid (g/mol), and V is the capacity of the chamber (L).
The equation (1) is used to compute the sensor's sensing response [34]
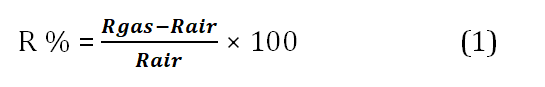
Where S is the sensing response; is resistance to gas and is the air resistance.
Accounting to the IUPAC, the sensitivity is defined as the slope of the graph plot between the X-axis (concentration) and Y-axis (sensing response) of the sensor is following equations and its unit will be ppm-1.
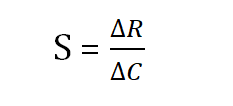
Where the Changes in sensor response and gas concentration are represented by ?R and ?C, respectively.
XRD analysis
X-Ray Diffraction (XRD) was used to analyze the crystal structure, phase identification, crystalline size and to identify the pattern of zeolite LTL, Zeolite Ag-LTL, and PPy/Zeolite Ag-LTL nanoparticles samples. The crystal phases were found with 2q values ranging from 2o to 90o. The diffraction peaks exemplify the pattern of zeolite LTL and Zeolite Ag-LTL, thus verifying the preparation of PPy/Zeolite Ag-LTL nanocomposite.
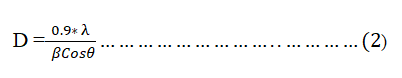
| Sample name | Crystalline size D (nm) | Lattice strain (e) |
|---|---|---|
| Zeolite – LTL | 35 | 0.0205 |
| Zeolite Ag– LTL | 25 | 0.0288 |
| Zeolite Ag– LTL/PPy | 32 | 0.0232 |
Table 1: Crystalline size and Lattice strain of the samples.
Each of the three samples has a different structure and various distinguishing peaks. Both Zeolite LTL and Ag-LTL samples had strong and prominent reflections on the XRD patterns (Fig. 2), indicating that the materials are extremely crystalline. In both samples (Zeolite LTL and Zeolite Ag-LTL) the reflections are indexed based on hexagonal crystal system which is showing good agreement with the LTL Zeolite structure [35]. The ion exchange in the zeolite with silver oxide and PPy does make a change in intensity only, not anything in zeolite structural morphology. “When” adding PPy to zeolite is affected the influence of intensity only not anything else.
The powder XRD patterns of zeolite LTL, Zeolite Ag-LTL, and PPy/Zeolite Ag-LTL nanoparticles are shown in Fig. 3. Scherrer's equation was used to calculate the average crystallite size of the PPy/Zeolite Ag-LTL nanocomposite:
D = )
Where D is crystallite size in nm; λ is a wavelength; β is full width at half maxima (FWHM), and θ is Bragg’s angle.
FT-IR analysis
FT-IR spectroscopy in the wavelength from 4000 to 500 cm-1, with resolution and increments, ware 461 cm-1 and 650 cm-1 respectively. The adsorption sites and structure of the zeolite samples were determined using infrared imaging. Zeolite and their composite physical were put onto a sample holder and allowed to pass light from the control of instruments. FTIR spectra (Fig. 3 (a) & (b)) of the observation band in the zeolite and modified zeolite structure were showing the absorption band at around 461 cm-1 and 613 cm-1. In 726 cm-1 and 776 cm-1, symmetric stretching bands were detected. In the 1027 cm-1 spectrum, the asymmetric stretch vibration bands were discovered. In 1643 cm-1 and 3639 cm-1, respectively, water-binding molecules and OH stretching bands were discovered.
The absorption peaks in zeolite composite were (600, 666, 713, 853, 965, 1025, 1160, 1296, 1450, 1543, 1102, 2342, and 2659) cm-1, corresponding to C-H bonding, a quinoid ring of vibration mode, C-N stretching of vibration, benzenoid ring stretching, N-Quinoid ring stretching, and N-H stretching vibrations, respectively. All these peaks were showing the presence of polypyrrole in the zeolite composite [36-37].
SEM analysis
The study's morphology of the synthesis parent zeolite and their composite related images are more clearly observed for better discussion and interpretation in Fig. 4 (a), (b) & (c). A regular hexagonal rod with an average length of 77.64 nm and a diameter of 23.52 nm is depicted in these photos. Agglomeration and aggregation crystals are visible and integrated due to the high surface charge [38]. The grains are represented in a regular pattern, and the microstructure is made up of many neatly aligned rod-shaped particles (nano-tablet tube). The shape of their composite is that of a cauliflower. PPy was encapsulating the zeolite, according to SEM pictures of Zeolite polypyrrole.
EDX analysis
Elemental analysis for Aluminum, Silicon, Oxygen, sodium, carbon, potassium, and silver in the synthesis of zeolite and composites were observed. The unit cell contains zeolite LTL is K9[Al9Si27O72]. H2O. From this EDX analysis (Fig. 5 (a) & (b)), it was found that the content of elements such as Si, O, Al, K in Zeolite LTL, Ag obtains of ion exchange Zeolite Ag-LTL and C content in zeolite polypyrrole composites. All elements contained in the sample was showing the synthesis successfully. Chlorine and iron element of the composite were not found because this sample washing repeatedly.
Sensing mechanism
Polypyrrole is a p-type semiconductor. There are obtaining owing basic structural properties which can interact readily with ammonia gas. Polypyrrole is a p-type semiconductor whose carrier is a hole. Because PPy is p-type, it reacts with reducing gases like ammonia, resulting in a drop in charge carrier density and conductivity when the charge carrier mobility diminishes. The sensitivity of undoped PPy is poor. When molecules are exposed to ammonia gas, they get physisorbed and undoped. The concentration of ammonia gas in the sensor is then increased, resulting in a larger carrier density and, as a result, a better response [39]. Ammonia is a redox gas that works as an electron donor; when PPy combines with gas, electric resistance drops dramatically. The resistance of the PPy can be measured after flashing with air or dry nitrogen gas. As a result, this process represents ammonia and PPy interactions [40]:
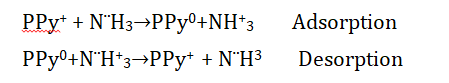
The interaction of PPy and Ammonia gas was first described by Gustafsson et al [41]. There are reported that when PPy was exposed to low concentration ammonia gas for short time, proton transfer occurs between PPy and Ammonia gas to form NH4+. This process was working on reversible at low concentration and the result show of decrease in conductivity. Trojanowicz et al [42] reported that PPy film was used in the fabrication sensor. There was showing a decreased response of ammonia gas at low concentrations and the proton transfer process is reversible. Furthermore, a nucleophilic attack on the carbon atom of PPy Backbones is common, which might result in irreversible PPy alterations. Lähdesmaki et al [43] reported that PPy material use for sensor makes. Lower concentrations of ammonia gas are not used whereas larger concentrations are used. So this problem is solving to take a new type of material to prepare gas sensor at working lower concentration of ammonia gas. Tiwari et al [44] reported that the PPy thin-film sensors show very good sensitivity to ammonia gas at lower to higher concentrations. So this is showing 0.37 % Sensitivity at lower concentrations (3 ppm).
In the present study, the fabrication sensor (PPy/Zeolite L) is exposed to different concentrations of ammonia gas (1 ppm, 2 ppm, 5 ppm). The resistivity of PPy/Zeolite Ag-LTL composites increases with ammonia gas exposure during ammonia gas sensing. When an ammonia molecule interacts with PPy/Zeolite Ag-LTL composites, the doublet of nitrogen in ammonia loses an electron to the nitrogen of the polymer backbone, forming the energetically more favorable ammonium ion NH4+ at the N+ -H adsorption center, which is similar to a de-doping (deprotonating) process; these electron transfers from ammonia to PPy/Zeo As the quantity of ammonia gas increases, the sensor's sensing response increases, as shown in Fig. 9. The sensor when flesh with normal air its docent reverses to the baseline which shows that the scant of the ammonia gas molecules are trapped inside the sensing materials. But after some peaks reverse to the baseline after on the fan which shows the complete recovery. As zeolite is introduced to PPy, its sensitivity to NH3 increases. This is because zeolites have extremely high surface areas due to their Three Dimensional (3D) frameworks with open porosity. As a result, adding zeolite to PPy increases its surface area, allowing more NH3 molecules to be absorbed and interact with the polymer chains. When compared to similar types of sensor material reported in earlier works (shown in Table 2).
| Sample | Operating temperature | Response Time | Recovery Time | Sensitivity | Concentration (Gas) | Ref. |
|---|---|---|---|---|---|---|
| polyaniline/zeolite erionite | RT | - | - | 5.33 | 2.5 ppm (NO2) | 45 |
| Pani/Zeolite-Mor(Si/Al=10) | 30 oC (+2) | 24 s | 28.5 s | 11 | 25 ppm (CEES) | 46 |
| Polypyrrole/Zeolite Ag-LTL | 30 oC (+2) | 15 s | 35 s | 12.34 | 1 ppm (NH3) | This work |
Table 2: Shows a comparison to a previously reported sensor made of comparable material.
Response and recovery time: The response and recovery time of the sensor are two crucial key elements for sensors, which are characterized as Response time is the time it takes to get from 90% of baseline to maximum when gas is present, and recovery time is the time it takes to get from 90% of maximum to minimum (baseline) when gas is absent. The response and recovery time of the sensor for NH3 were measured, and the findings are given in Figures (6) and (7). At 1 ppm, the sensor's reaction and recovery times are 15 and 35 seconds, respectively.
Sensitivity:
The sensitivity of a sensor refers to how little a change in concentration can detect. The change in resistance when exposed to a target gas relative to the baseline resistance is how a sensor's sensitivity (S) is expressed. The formula below was used to calculate the sensitivity of the sensors.
S = [ ] *100
Where Rg and Ra are the film resistances during NH3 gas exposure and air exposure, respectively. The sensing response vs concentration of PPy/Zeolite-LTL is presented in Fig. 8. The manufactured sensor's sensor sensitivity was discovered to be 12.34 ppm-1.
Selectivity:
Selectivity refers to a sensor's capacity to differentiate one gas from another when many gases are present. The manufacturing sensor is shown in Fig. 9 to determine the sensor's selectivity for NH3 gas. The sensor response to ammonia is the highest as compared to other gases, as seen in the bar diagram. This shows that the fabrication sensor is selective for NH3, and it will be utilized to create a real-time gas sensor that can detect NH3 in the absence of other gases with concentrations of 1 ppm or less, such as xylene, benzene, carbon monoxide, and nitrous oxide.
Humanity:
The performance of an ammonia gas sensor can be influenced by humidity. The conditions used in these studies were the same as those used in the 5 ppm NH3 analysis. Inside the chamber, selected aliquots of water were evaporated. At specific times, the sensor's resistance was measured. The research was expanded to look into the impact of a 55 percent increase in RH.
Methylene blue dye degradation
Methylene blue dye in various concentrations was made (10 ppm, 15 ppm, 20 ppm, and 25 ppm). The prepared concentrations were subjected to UV-visible photo spectroscopy, as illustrated in Fig. 10. (a) In Fig. 10 (b) the graph was a plot between concentrations vs absorption and linear fitting was done. The degradation percentage was calculated as
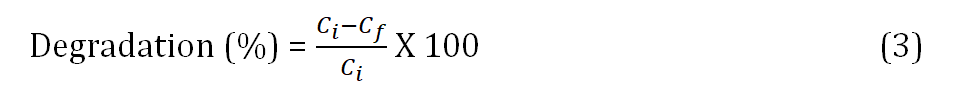
To 20 ppm of methylene blue (MB) dye, 2 mg of Zeolite polypyrrole nanocomposite was added. The setup was done in full darkness with UV light shining on it (11 W). UV-visible photo spectroscopy was performed at various time intervals (0 min, 15 min, 30 min, 45 min, 60 min, 75 min, and 90 min). It was seen that as the time of exposure increases, the rate of dye degradation increases (absorption decreases), as shown in Fig. 10 (c) and (d). After 90 min the dye degradation was 79 %. As a result, the polymer nanocomposite developed can remove the dye from contaminated water.
In summary, Zeolite Nanoparticle was synthesis via the hydrothermal method, and PPy/Zeolite Ag-LTL was prepared using the Polymerization method and characterized by FTIR, XRD, EDX, and SEM. The intensity and sharp peaks determine the highly crystalline structure of the XRD pattern confirm by Zeolite Nanoparticle and PPy/Zeolite composite. The Hexagonal and Cauliflower patterns may be seen in the SEM micrographs of Zeolite and PPy/Zeolite Composite, respectively. The size of Zeolite nanoparticles regular hexagonal rod has an averaging length of 77. 64 nm, diameter 23.54 nm, and the average crystallite size was approximately 35 nm. The constructed sensor was capable of detecting ammonia gas concentrations as low as 1 ppm. The constructed sensor had a response and recovery time of less than a minute. Methylene blue can be degraded by the polymer composite up to 73 percent at 20 ppm. Thus, the polymer nanocomposite shows the dual function sensor as well as dye degradation.
Conflict of interest
The authors declare that they have no known competing financial interests or personal ties that could have influenced the research presented in this study.
Acknowledgments
The authors are grateful to Jiwaji University's School of Studies in Physics and Chemistry for providing the experimental facilities. The authors thank Jiwaji University's Central Instrumentation Facility Laboratory (CIFL) in Gwalior for the XRD and FTIR research, as well as IIT Roorkee for the SEM and EDX study.
Citation: Dandotia A , Dipak P, Dandolia RKS, Tiwari RK, T omar R, e t al. (2021) Studies on the s yn thesis and Characterization of Zeolite-LTL/PPy Composite for Gas Sensing Application. Polym Sci Vol.7 No.1
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.