Keywords
Au nanoparticles; Multiwall carbon nanotubes; Man-Os (VI) tmen polymer; Dopamine; Poly(Sodium 4-styrene sulfonate)
Introduction
Today, awareness on the levels of the important species in the body is one of the main necessities for everyone in order to control health and increase quality of life. Therefore development of a reliable, simple-sensitive, and selective detection methodology has always been of interest for many researchers in this area. In order to achieve these goals, electrochemical methods along with chemically modified electrodes (CMEs) have been successfully employed [1]. The selectivity and sensitivity of the CMEs can be improved easily by choosing a suitable modifier compared to the equivalent bare ones [2,3]. During past decades and later on, many different types of modifiers have been used to build up a desired chemical functionality on the electrode surface, including conducting and non-conducting polymers, ionic liquids, redox organic and inorganic mediators [4-13]. However, among these modifiers, conducting and non-conducting polymers have been extensively used as transducers in biological sensors owning to their unique electrical, electronic, favorable permselective and physical properties [14-17]. Also, nanomaterials were commonly used in this field according to their excellent electronic and electrocatalytic properties, which enhance the rate of electron transfer between the electrode surface and the reactive species in the reaction medium [18-23]. Many studies have been reported on the application of carbonaceous electrodes modified with different nanoparticles such as gold, platinum, palladium, nickel, copper, and silver [24-28]. Gold nanoparticles have been in the forefront of these nanoparticles, due to their highly catalytic performance and unique prosperities [29-31]. On the other hand carbon nanotubes have also been interesting as electrode materials according to their unique physicochemical properties and high surface area. Therefore, it seems that one can achieve much better catalytic effects by combining metal nanoparticles and carbon nano-tubes compared to these materials alone [32]. These hybrid nanomaterials have received much more attention according to their interesting structural, electrochemical, electromagnetic and other properties, which led to use successfully in the construction of the sensing devices [33-40].
Dopamine or (3,4-dihydroxyphenylethylamine, simply denoted as DA), is an important neuro-transmitter, which is mainly distributed in the mammalian central nervous system and plays an essential role in the function of the central nervous, renal, hormonal and cardiovascular systems [41]. Concentration of plasma DA normally are in the range between 10-8 M and 10-6 M, but the primal DA concentration is very low (0.01-1 μM) in the extra- cellular fluid of the central nervous system [42], the deficiency of the DA level lower than the normal leads to neurological disorders like Schizophrenia and Parkinson’s disease [43,44]. Different methods for DA detection have been studied such as detection with a molecular imprinted polymer MIP-hybrid sensor [45]. Or a sensor for DA and AA fabricated by copper dispersed sol-gel composite electrode using (3-mercaptopropyl) trimethoxysilanes and so on. Among various analytical techniques, electrochemical strategy for DA detection in biological samples has attracted more interest because of the features such as high sensitivity, tunable selectivity, low detection limits and fast response [46-48]. DA can be detected by electrochemical oxidation reaction however, electro oxidation of DA occurs at relatively high potentials on common electrodes, where other co-existing compounds such as ascorbate (AA) and urate (UA) can be also oxidized simultaneously [42]. The oxidation waves of these compounds are located in the same potential range and overlap with that of DA, and either decrease or prevent the selective determination of DA [44,49-51]. These problems have been solved by choosing a suitable modification strategy on the basis of enhancement of electron transfer rate and/or electrostatic repulsion of mentioned interferences. A wide variety of modifiers have been used for these purposes including: selfassembled monolayers, gold nanoparticles, carbon nanotubes, conducting or electrochemically generated polymers, fabricated microelectrode arrays (MEA) chip and carbon nanotubes at ITO [52], multifunctional MEA chips [53] and such that many others different modifiers with different strategies have been reported in this field [44,48,50,52,54-56]. In the present work, the adducts comprising 3α,6α- mannan attached to the six valent–osmium complex with N,N,N΄,N΄-tetramethylethylenediamine [Man- Os (VI) tmen] was used as electrocatalyst for oxidation of DA, because of its promising prospects in the electron–transfer, selectivity, wide electrochemical potential window, and electrical conductivity [57,58]. Man-Os (VI) tmen was immobilized on the surface of graphite electrodes via a simple adsorption route and then covered with poly (sodium 4-styrene sulfonate) (PSS) in order to achieve high analytical features. The sensor was further modified with multiwall carbon nanotubes decorated with gold nanoparticles (Au-NPs-MWCNTs). The electrochemical behaviors of the resulting sensors were investigated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Both modified electrodes showed high electro catalytic activity towards oxidation of DA even in the presence of high concentrations of AA and UA as interfering compounds. The proposed sensors exhibited low detection limit, high sensitivity and wide linear dynamic range.
Materials and Methods
Chemicals
All chemicals were Lyophilized yeast mannan (Man) from Saccharomyces cerevisiae (Sigma cat. no. M7504, MW in range from 34,000 to 62,500 Da) purchased from Sigma Aldrich and were of analytical grade. Dopamine hydrochloride from (Aldrich, Steinheim, Germany), ascorbic acid were purchased from ICN Biomedical Inc (Malmö-Stockholm, Sweden). Uric acid was purchased from Sigma-Aldrich (St. Louis, MO, USA). Gold (III) acetate, 99.9% (metal basis) powder was from Alfa Aesar. Multiwall carbon nanotubes (95% purity, OD=10-30 nm, ID=5-10 nm, and length=0.5-500 μm) were obtained from Aldrich. Poly (sodium-4 styrene sulfonate) (PSS) with an average MW of 70000 30% in H2O was purchased from Sigma-Aldrich and was used with 15% concentration. Sodium dihydrogen phosphate and disodium hydrogen phosphate were purchased from Sigma- Aldrich and Merck (Darmstadt, Germany) respectively. All aqueous solutions were prepared with purified water in a Milli-Q water purification system (Millipore. Bedford, MA, USA).
Apparatus
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were performed using an Autolab potentiostat (Model PGSTAT30, Utrecht, The Netherlands) with a conventional three-electrode set-up, in which graphite modified electrodes, an Ag|AgCl|(KCl sat.) electrode, a platinum rod served as the working, reference, and auxiliary electrodes respectively, and the output signal was acquired by Autolab GPES software (version 4.9).
Transmission electron microscopy (TEM) measurements were performed using a normal TEM- instrument, (1230 JEOLTEM, Japan) at 80 kV. Scanning electron microscopy (SEM) measurements were done using DEM-instrument, Hitachi SU3500, at secondary electron detector (SE-detector), 5 kV in a high vacuum. All measurements were achieved at room temperature. Prior to the experiments, the solutions were purged with argon for 20 min to remove dissolved oxygen.
Preparation of Man-Os (VI) tmen
Man-Os (VI) tmen was prepared by mixing potassium osmate dihydrate with free N,N, N´,N΄-tetramethylenediamine (tmen) in 1:1 molar ratio in water solution, followed by adjustment of pH to 7, to produce Os(VI)tmen. The modification of 3α,6α– mannan (Man) was achieved as described in [58] according to the reaction of 5 mM Man with 5 mM Os (VI) tmen in 50 mM phosphate buffer at pH 7 for 16 h with agitation at 37°C. Man-Os (VI) tmen was dissolved in 0.1 M PBS at pH 7 at concentration of 0.02 mg. μl-1 to be used for modification of graphite electrodes.
Preparation of Au-NPs-MWCNTs
The MWCNTs were decorated with gold nanoparticles (AuNPs) according to a method reported previously [59] with some modifications. The treatment of MWCNTs was conducted by refluxing with 70% HNO3 for 16 h, followed by filtering and thorough washing of the materials with deionized water until pH 7 and then dried in a vacuum oven. The pretreatment of the MWCNTs (equivalent to 8 mmol of carbon) were dry mixed with 0.8 mmol of Au(CH3COO)3 using a mortar and pestle for 30 min under ambient conditions and then transferred to a glass vial and heated in an oven to 300°C, for 1 h and held isothermally for 3 h under nitrogen, the product was then collected as the Au-NPs-MWCNTs. In this study, the time applied for dry mixing of the pretreated MWCNTs with powdered Au(CH3COO)3 was at least 15 min more than the time applied in the method reported previously (10-15 min) [59].
Fabrication of the modified electrodes
Prior to the modification processes, bare graphite rods (Alfa Aesar GmbH and Co KG. Karlsruhe, Germany, AGKSP grade, ultra “F” purity, diameter; 3.05 mm, geometric surface area 0.073 cm2) were polished with wet emery SiC paper (Tufback Durite, P1200), and then rinsed thoroughly with Milli-Q-water. After being dried, 2 μl of a 0.02 mg.μl-1 Man-Os (VI) tmen solution was cast on the polished end of the graphite electrode (GE) and then the surface of GE/Man-Os (VI) tmen was then covered with 1 μl of 15% water solution of PSS and allowing it to dry at room temperature (denoted as GE/Man-Os (VI) tmen/PSS). In the second procedure of modification, the graphite electrode was firstly modified with Au-NPs-MWCNTs via a simple casting of 6 μl of a well-dispersed 1 mg ml-1 dimethylformamide solution on the surface of electrode. After adsorption, 2 μl of a 0.02 mg.μl-1 Man-Os (VI) tmen solution were cast on the Au-NPs-MWCNTs modified graphite electrode (denoted as GE/Au-NPs-MWCNTs/Man-Os (VI) tmen). Finally the modified electrode was dried at room temperature for about 20 min.
Results and Discussion
Characterization and electrochemical behavior of GE/Man-Os (VI) tmen
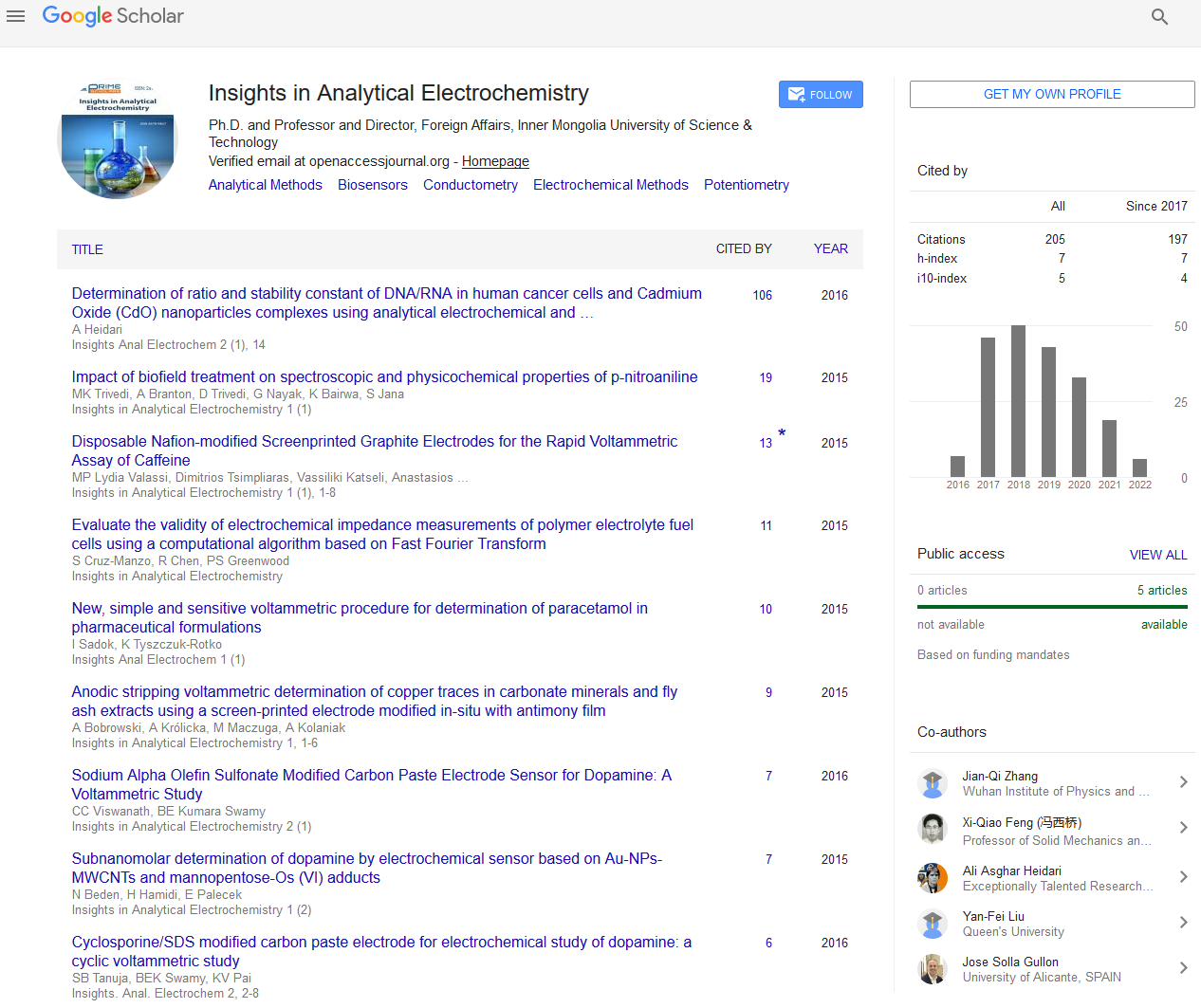
The electrochemical properties of the Man-Os (VI) tmen have previously been described in [57,58], where the formal potential of adducts-polymer E0’ was reported to -220 mV vs. Ag|AgCl| (KClsat.) The electrocatalytic behavior of the Man-Os (VI) tmen modified graphite electrode was evaluated thoroughly for the oxidation of DA, AA, and UA. In the recent work (Figure 1) shows the CVs of the bare GE in the absence (a: cyan color) and presence (c: green color) of 1 mM DA in 0.1 M PBS buffer pH 7. As it can be seen, no redox peaks were observed on the bare electrode in the absence of DA, while a couple of peaks were observed in the presence of this electroactive compound. The formal potential E°' was found to be 184 mV vs. Ag|AgCl| (KClsat.). This observation is in consensus with results reported by others (Figures 2A and 2B) [60] demonstrates the CVs of the Man-Os (VI) tmen modified graphite electrode in the absence (b: violet color) and presence (d: red color) of 1 mM DA, respectively. By comparison of Figure 2A with Figure 2B, it can be seen that the anodic peak current (Ia=47.88 μA) of DA on the Man-Os (VI) tmen modified GE is higher than that on the bare electrode (Ia=29.57 μA) with a negative shift in the anodic peak potential. This observation confirms the predicted electrocatalytic behavior of the Man-Os (VI) tmen modified electrode. However slight electrocatalytic effects were observed for AA and UA at the Man-Os (VI) tmen modified electrode at same condition (See Figures 2A and 2B). The observed behavior might be attributed to the good diffusion of DA into the film of the Man-Os (VI) tmen on the modified electrode, because DA has an aromatic ring structure with resonance hybrid. Therefore, the intermediate state of oxidized DA can be easily stabilized through an electrostatic interaction with the Man-Os (VI) tmen redox center, while AA and UA are not aromatic compounds. These obtained results explain clearly the excellent electrocatalytic effect of Man-Os (VI) tmen modified electrode towards the oxidation of DA
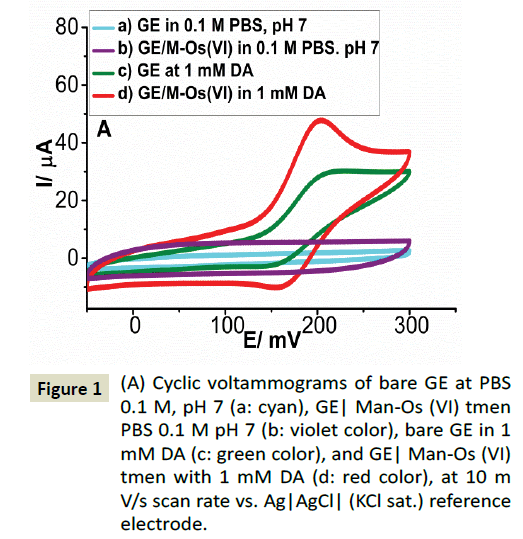
Figure 1: (A) Cyclic voltammograms of bare GE at PBS 0.1 M, pH 7 (a: cyan), GE| Man-Os (VI) tmen PBS 0.1 M pH 7 (b: violet color), bare GE in 1 mM DA (c: green color), and GE| Man-Os (VI) tmen with 1 mM DA (d: red color), at 10 m V/s scan rate vs. Ag|AgCl| (KCl sat.) reference electrode.
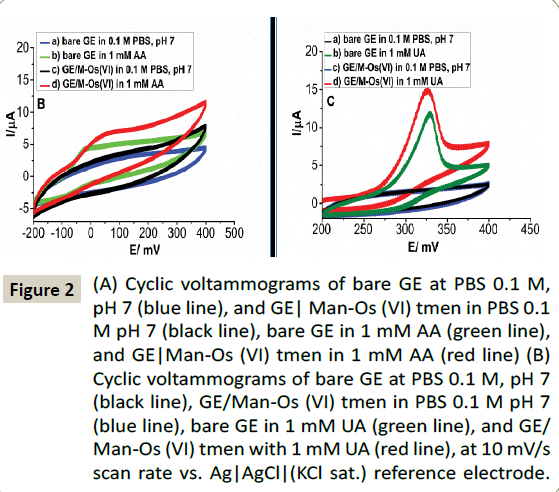
Figure 2: (A) Cyclic voltammograms of bare GE at PBS 0.1 M, pH 7 (blue line), and GE| Man-Os (VI) tmen in PBS 0.1 M pH 7 (black line), bare GE in 1 mM AA (green line), and GE|Man-Os (VI) tmen in 1 mM AA (red line) (B) Cyclic voltammograms of bare GE at PBS 0.1 M, pH 7 (black line), GE/Man-Os (VI) tmen in PBS 0.1 M pH 7 (blue line), bare GE in 1 mM UA (green line), and GE/ Man-Os (VI) tmen with 1 mM UA (red line), at 10 mV/s scan rate vs. Ag|AgCl|(KCl sat.) reference electrode.
Selective determination of DA at the GE/Man-Os (VI) tmen/PSS
The capability of the proposed sensor to resolve the oxidation peak of DA from the peaks of AA and UA was evaluated by differential pulse voltammetry of (DPV). In Scheme 2B demonstrates the voltammograms the (a) bare GE, (b) the GE/ Man-Os (VI) tmen and (c) GE/ Man-Os (VI) tmen/PSS in a mixed solution containing 5 mM, 0.5 mM, 100 μM of AA, UA and DA, respectively. The concentration ratios of AA and UA to DA were the same as in real blood samples. As can be seen, all these compounds were oxidized at bare GE (Figure 3A) and GE/Man- Os (VI) tmen (Figure 3B). The peaks correspond to AA, DA and UA at the both electrodes, respectively, from left to right. In order to specify the peak position of these compounds, the DPVs of each of them were recorded at both electrodes in separate solutions (See Figure 3A). Note that the peak heights for all of these compounds at the GE/Man-Os (VI) tmen were higher than at bare GE. These observations beside the results of CVs studies confirm the electrocatalytic effect of Man-Os (VI) tmen adducts on the oxidation of these compounds, especially for DA.
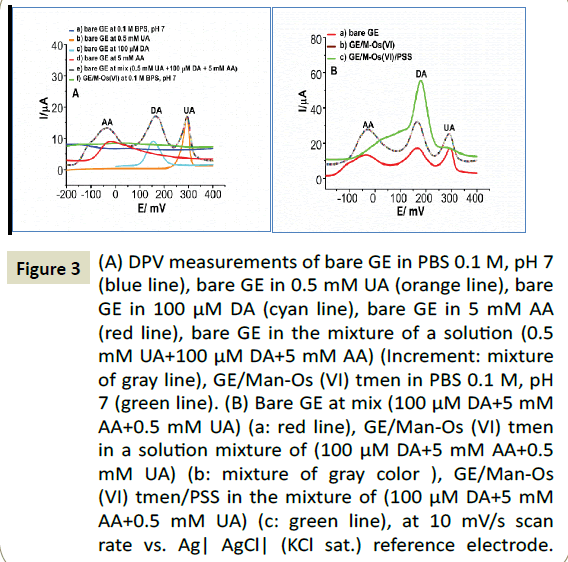
Figure 3: (A) DPV measurements of bare GE in PBS 0.1 M, pH 7 (blue line), bare GE in 0.5 mM UA (orange line), bare GE in 100 μM DA (cyan line), bare GE in 5 mM AA (red line), bare GE in the mixture of a solution (0.5 mM UA+100 μM DA+5 mM AA) (Increment: mixture of gray line), GE/Man-Os (VI) tmen in PBS 0.1 M, pH 7 (green line). (B) Bare GE at mix (100 μM DA+5 mM AA+0.5 mM UA) (a: red line), GE/Man-Os (VI) tmen in a solution mixture of (100 μM DA+5 mM AA+0.5 mM UA) (b: mixture of gray color ), GE/Man-Os (VI) tmen/PSS in the mixture of (100 μM DA+5 mM AA+0.5 mM UA) (c: green line), at 10 mV/s scan rate vs. Ag| AgCl| (KCl sat.) reference electrode.
In order to improve the selectivity and to increase the sensitivity of the sensor for determination of DA, the modified electrode was covered further with poly (sodium-4 styrene sulfonate) (PSS). In consequence of this modification step, the electrode surface becomes negative because of the sulfonate groups of the PSS [61,62]. Since the values of the acidic dissociation constant (pKa) for DA, AA, and UA are respectively [63,64], Therefore, AA and UA are deprotonated at pH 7, while DA remains in its acidic form. Figure 4 demonstrates the DPV of the GE/Man-Os (VI) tmen/PSS in the solution containing all compounds. As it can be seen, not only the peaks of AA and UA were decreased but also the peak height of DA was increased. This observation confirms that AA and UA are repelled from the surface of the electrode and DA attracted by the sulfonate groups, resulting in an enhancement in the electron transfer rate, either via strong π-π interaction, or due to the favorable electrostatic interaction. On the basis of the obtained results, the analytical characteristic of the GE/ Man-Os (VI) tmen/PSS were evaluated for determination of DA in the presence of an excess concentration of AA (5 mM) and UA (0.5 mM) by DPV technique. Figure 4A illustrates a typical DPVs and a calibration curve (Figure 4B) obtained for dopamine using GE/Man-Os (VI) tmen/PSS, at different concentrations of DA (0.1 nM to 100 μM) in the presence of an excess concentration 5 mM AA and 0.5 mM UA. The current density was linear with concentration of DA in the range between 0.1 nM and 20 μM with a correlation coefficient of 0.9963. The sensitivity and the limit of detection (3SD/slope) for DA were 358 μA cm-2μΜ-1 and 2.8 nM, respectively. The higher current density (J) was 959 ± 2.86 μA.cm2.
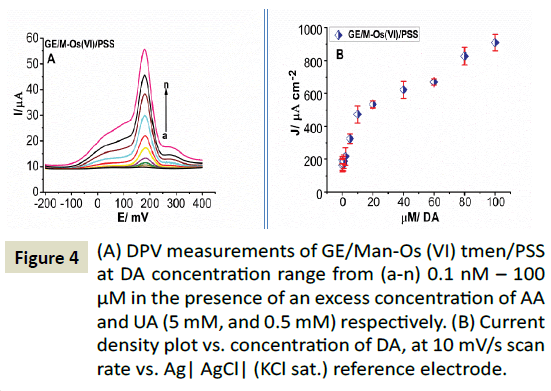
Figure 4: (A) DPV measurements of GE/Man-Os (VI) tmen/PSS at DA concentration range from (a-n) 0.1 nM – 100 μM in the presence of an excess concentration of AA and UA (5 mM, and 0.5 mM) respectively. (B) Current density plot vs. concentration of DA, at 10 mV/s scan rate vs. Ag| AgCl| (KCl sat.) reference electrode.
Selective determination of DA, at GE/Au-NPs- MWCNTs/Man-Os (VI) tmen
In an effort to improve the sensitivity of the proposed DA sensor, the electrode was further modified by gold nanoparticles decorated multiwall carbon nanotubes (denoted GE/Au-NPs- MWCNTs/Man-Os (VI) tmen). It has been expected that this hybrid nanomaterials will increase the surface area of the electrode and catalytic properties of the Man-Os (VI) tmen. Firstly, the AuNPs-MWCNTs were characterized by transmission electron microscopy (Figure 5A) with difference magnifications (A, B, C) which revealed the homogenous formation of AuNPs (black dots in the image) on the walls of the carbon nanotubes, and some bigger dots attributed to the aggregation of AuNPs with a diameter of approximately 50 nm these results are in with the literature [59]. After modification of graphite electrode by the Au-NPs-MWCNTs its surface was analyzed by scanning electron microscopy (Figure 5B) with difference magnifications (A, B, C, D). The SEM images of the GE/Au-NPs-MWCNTs was revealed the existence of gold nanoparticles on the surface of the MWCNTs same as TEM image. The TEM and SEM images prove that there is a strong tangle between the AuNPs and MWCNTs as a result of electrostatic interactions [59]. Also, the morphology of the GE/AuNP-MWCNT/MAN -Os (VI) tmen was studied by SEM and a porous structure of the modified layer on the surface of the electrode was observed after deposition of the Man-Os (VI) tmen (Figure 5C) at different magnifications (A, B, C). AuNPs with a spherical shape attached to the MWCNTs were distributed evenly in the Man-Os (VI) tmen matrix at the entire region of the electrode surface.

Figure 5A: (A) TEM–images for Au-NPs decorated MWCNT 1 mg/ml solution. At different magnification (A) 400 X. (B) 15 X and (C) 200 X.

Figure 5B: (B) SEM-images of the GE/AuNPs-MWCNTs with different magnification, (A) 500 X, (B) 5000 X, (C) 21000 X, (D) 37000 X.
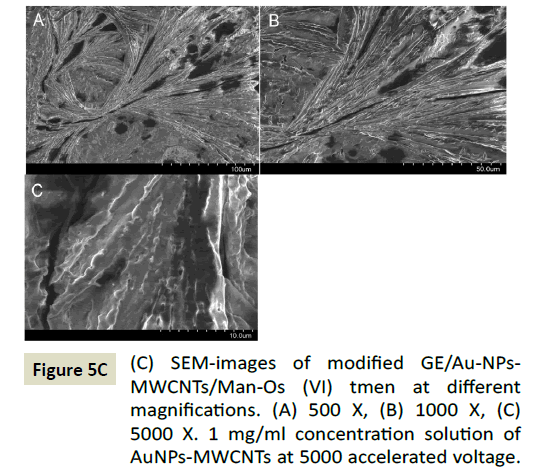
Figure 5C: (C) SEM-images of modified GE/Au-NPs- MWCNTs/Man-Os (VI) tmen at different magnifications. (A) 500 X, (B) 1000 X, (C) 5000 X. 1 mg/ml concentration solution of AuNPs-MWCNTs at 5000 accelerated voltage.
The catalytic properties of the GE/Au-NPs-MWCNT/Man-Os (VI) tmen was evaluated in 0.1 M PBS buffer solution pH 7 containing 100 μM DA by cyclic voltammetry (Figure 6A and 6B). For comparison purpose, the CV of GE/Man-Os (VI) tmen was also recorded at same condition (Figure 6A and 6B). As can be seen in a Figure 6 the electrode modified by the Au-NPs-MWCNTs shows a higher anodic current for DA oxidation and lower potential. This observation proved the combined catalytic effect of hybrid nanomaterial and Man-Os (VI) tmen adducts.
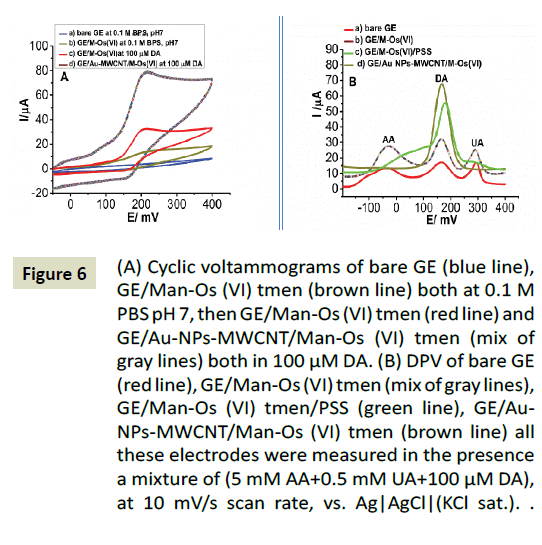
Figure 6: (A) Cyclic voltammograms of bare GE (blue line), GE/Man-Os (VI) tmen (brown line) both at 0.1 M PBS pH 7, then GE/Man-Os (VI) tmen (red line) and GE/Au-NPs-MWCNT/Man-Os (VI) tmen (mix of gray lines) both in 100 μM DA. (B) DPV of bare GE (red line), GE/Man-Os (VI) tmen (mix of gray lines), GE/Man-Os (VI) tmen/ PSS (green line), GE/Au-NPs-MWCNT/Man-Os (VI) tmen (brown line) all these electrodes were measured in the presence a mixture of (5 mM AA+0.5 mM UA+100 μM DA), at 10 mV/s scan rate, vs. Ag|AgCl|(KCl sat.) reference electrode.
Moreover, in order to study the effect of interfering compounds (AA and UA), differential pulse voltammograms of the (a) bare GE, (b) GE/Man-Os (VI) tmen, (c) GE/Man-Os (VI) tmen/PSS, (d) GE/ AuNPs-MWCNTs/Man-Os (VI) tmen, were recorded in 0.1 M PBS buffer solution pH 7 containing of AA (5 mM), UA (0.5 mM) and DA (100 μM) (Figure 6B). This study showed that the peak current of DA oxidation at the (d) GE/AuNPs-MWCNTs/Man-Os (VI) tmen, was higher than those observed at the other three electrodes. It seems that the electrode modified by the AuNPs-MWCNTs provides a unique surface area, and produces a suitable gallery to gather a large number of DA molecules on the electrode surface. Also this hybrid nanomaterial increases the electron transfer rate between the electrode and DA. In addition, no peaks were observed for AA and UA at the GE/Au-NPs-MWCNTs/Man-Os (VI) tmen, which was an extraordinary result. Therefore, the GE/Au- NPs-MWCNTs/Man-Os (VI) tmen could be successfully utilized for selective detection of DA in the solution containing interfering compounds.
Finally, the GE/AuNPs-MWCNTs/Man-Os (VI) tmen was used to determine DA in the presence of an excess concentration of AA (5 mM) and UA (0.5 mM) by the DPV technique (Figure 7A). The plot of current density versus DA concentration was constituted of two linear segments with different slopes, corresponding of two different DA concentration ranges 1 pM - 500 pM and 0.5 nM - 40 μM with correlation coefficients of 0.9991 (Figure 6B). The sensitivity and the limit of detection (3SD/slope) for DA were 624.49 μA cm-2μΜ-1 and 0.17 pM respectively. The higher current density (J) was 1475 ± 3.45 μA.cm2. Our results display a significant performance in comparison with different modification processes that have been reported in [65] for DA detection.
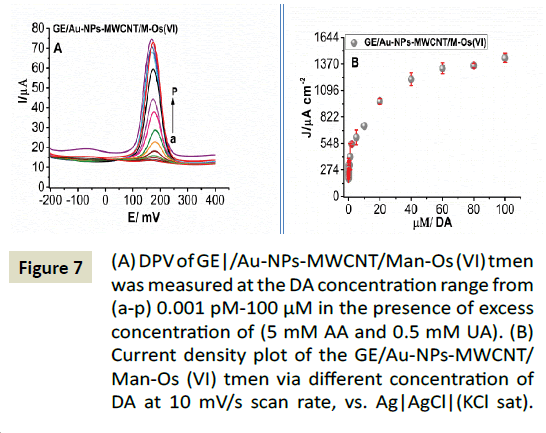
Figure 7: (A) DPV of GE|/Au-NPs-MWCNT/Man-Os (VI) tmen was measured at the DA concentration range from (a-p) 0.001 pM-100 μM in the presence of excess concentration of (5 mM AA and 0.5 mM UA). (B) Current density plot of the GE/Au-NPs-MWCNT/Man-Os (VI) tmen via different concentration of DA at 10 mV/s scan rate, vs. Ag|AgCl|(KCl sat) reference electrode.
Conclusion
The present work shows the application graphite electrodes modified by Au nanoparticles decorated multiwall carbon nanotubes (AuNPs-MWCNTs) and adducts comprising 3α,6α- mannan attached to the six-valent osmium complex with N,N, N´,N΄-tetramethylethylenediamine (Man-Os (VI) tmen) for determination of DA, because of their promising prospects in electron transfer, wide electrochemical potential window and electrical conductivity. The Man-Os (VI) tmen was immobilized on graphite electrode via a simple adsorption route and then covered by poly (sodium 4-styrene sulfonate) (PSS). In order to achieve high analytical features, the sensor was further modified with multiwall carbon nanotubes decorated with gold nanoparticles (Au-NPs-MWCNTs), The electrochemical behavior of the resulting sensors were investigated using voltammetry (CV) and differential pulse voltammetry (DPV). Both modified electrodes showed high and selective electrocatalytic activity towards oxidation of DA even in the presence of high concentrations of AA and UA as interference compounds.
Acknowledgements
This work was financially supported by Department of Biochemistry and Structural Biology, Lund University and the Swedish Research Council (Vetenskapsrådet, Project 2010-5031). The authors also thank Dr. Somayyeh Bozorgzadeh, Islamic Azad University, Iran for her support to this work.
References
- Oztekin Y, Tok M, Nalvuran H, Kiyak S, Gover T, et al. (2010) Electrochemical modification of glassy carbon electrode by poly-4-nitroaniline and its application for determination of copper(II). Electrochim Acta 56:387-395.
- Nazemi Z, Shams E, Amini MK (2010) Covalent modification of glassy carbon electrode by Nile blue: Preparation, electrochemistry and electrocatalysis. Electrochim Acta 55:7246-7253.
- Ma Q, Ai S, Yin H, Chen Q, Tang T (2010) Towards the conception of an amperometric sensor of L-tyrosine based on hemin/PAMAM/MWCNT modified glassy carbon electrode. Electrochim Acta 55:6687-6694.
- Chernyshov DV, Shuedene NV, Antipova ER, Pletnev IV (2008) Ionic liquid-based miniature electrochemical sensors for the voltammetric determination ofcatecholamines. Anal Chim Acta 621:178-184.
- Shvedene NV, Chernyshov DV, Pletnev IV (2008) Ionic liquids in electrochemical sensors. Russ J Gen Chem 78:2507-2520.
- Franzoi AC, Brondani D, Zapp E, Moccelini SK, Fernandes SC, et al. (2011) Incorporation of Ionic liquids in the construction of electrochemicalsensors. Quimica Nova 34:1042-1050.
- Safavi A, Maleki N, Honarasa F, Tajabadi F, Sedaghatpour F (2007) Ionic liquids modify the performance of carbon based potentiometric sensors. Electroanalysis 19:582-586.
- Zhu T, Row KH (2012) Monolithic materials and their applications in HPLC for purification and analysis of bioactive compounds from natural plants: a review. Instrum Sci Technol 40:78-89.
- Behpour M, Ghoreishi SM, Honarmand E, Salavati-Niasari M (2011) A novel N,N'-1,1'- dithiobis(phenyl)bis(salicylaldimine) self-assembled gold electrode for determination of dopamine in the presence of high concentration of ascorbic acid. J Electroanal Chem 653:75-80.
- Atta NF, Galal A, Ahmed RA(2011) Poly(3,4-ethylene-dioxythiophene) electrode for the selective determination of dopamine in presence of sodium dodecyl sulfate. Bioelectrochemistry 80:132-141.
- Balamurugan A, Chen SM (2007) Poly(3,4-ethylenedioxythiophene-co-(5-amino-2-naphthalenesulfonic acid)) (Pedot-Pans) film modified glassy carbon electrode for selective detection of dopamine in the presence of ascorbic acid and uric acid. Anal Chim Acta 596:92-98.
- Xue Y, Zhao H, Wu ZJ, Li XJ, He YJ, et al. (2013) Poly(pyrocatechol-3,5-disodiumsulfonate)/multi-walled carbon nanotubes composite forSimultaneous determination of dopamine, ascorbic acid and uric acid. J NanosciNanotechnol13:1563-1568.
- Kumar SP, Manjunatha R, Venkatesha TV, Suresh GS (2013) Polystyrene sulphonate wrapped multiwalled carbon nanotubes modified graphite electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Russ J Electrochem 49:299-306.
- Miao Y, Chen J, Hu Y (2005) Electrodeposited nonconductingpolytyramine for the development of glucose biosensors. Anal Biochem 339:41-45.
- ManiharSitumorang JJG, BrynnHibbert D (1999) Immobilisation of enzyme throughout a polytyraminematrix:a versatile procedure for fabricating biosensors. Anal Chim Acta 394:211-223.
- Yuqing M, Jianrong C, Xiaohua W (2004) Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol 22:227-231.
- Kausaite-Minkstimiene A, Mazeiko V, Ramanaviciene A, Ramanavicius A (2011) Evaluation of amperometric glucose biosensors based on glucose oxidase encapsulated within enzymatically synthesized polyaniline and polypyrrole. Sens Actuators B Chem 158:278-285.
- Heli H, Hajjizadeh M, Jabbari A, Moosavi-Movahedi AA (2009) Fine steps of electrocatalytic oxidation and sensitive detection of some amino acids on copper nanoparticles. Anal Biochem 388:81-90.
- Yang S, Liu X, Zeng X, Xia B, Gu J, et al. (2010) Fabrication of nano-copper/carbon nanotubes/chitosan film by one-step electrodeposition and its sensitive determination of nitrite. Sens Actuators B Chem 145:762-768.
- Shahrokhian S, Mahdavi-Shakib A, Ghalkhani M, Saberi RS (2012) Gold electrode modified with self-assembled monolayer of cysteamine-functionalized MWCNT and Its application in simultaneous determination of dopamine and uric acid. Electroanalysis 24:425-432.
- Atta NF, Galal A, El-Ads EH (2012) Gold nanoparticles-coated poly(3,4-ethylene-dioxythiophene) for the selective determination of sub-nano concentrations of dopamine in presence of sodium dodecyl sulfate. Electrochim Acta 69:102-111.
- Babaei A, Aminikhah M, Taheri AR (2013) A multi-walled carbon nano-tube and nickel hydroxide nano-particle composite-modified glassy carbon electrode as a new sensor for the sensitive simultaneous determination of ascorbic acid, dopamine and uric acid. Sens Lett 11:413-422.
- Yogeswaran U, Chen SM (2008) Multi-walled carbon nanotubes with poly(methylene blue) composite film for the enhancement and separation of electroanalytical responses of catecholamine and ascorbic acid. Sens Actuat B130:739-749.
- Tashkhourian J, Nezhad MRH, Khodavesi J, Javadi S (2009) Silver nanoparticles modified carbon nanotube paste electrode for simultaneous determination of dopamine and ascorbic acid. J Electroanal Chem 633: 85-91.
- Galal A, Atta NF, El-Ads EH (2012) Probing cysteine self-assembled monolayers over gold nanoparticles - Towards selective electrochemical sensors. Talanta93:264-273.
- Welch C, Compton R (2006) The use of nanoparticles in electroanalysis: a review. AnalyBioanal Chem 384:601-619.
- Li S, Zhu X, Zhang W, Xie G, Feng W (2012) Hydrogen peroxide biosensor based on gold nanoparticles/thionine/gold nanoparticles/multi-walled carbon nanotubes-chitosans composite film-modified electrode. Appl Surf Sci 258:2802-2807.
- La DD, Kim CK, Jun TS, Jung Y, Seong GH, et al. (2011) Pt nanoparticle-supported multiwall carbon nanotube electrodes for amperometric hydrogen detection. Sens Actuat B 155:191-198.
- German N, Ramanavicius A, Voronovic J, Oztekin Y, Ramanaviciene A (2011) The effect of colloidal solutions of gold nanoparticles on the performance of a glucose oxidase modified carbon electrode. Microchim Acta 172:185-191.
- Pingarrón JM, Yáñez-Sedeño P, González-Cortés A (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848-5866.
- Ramanaviciene A, Nastajute G, Snitka V, Kausaite A, German N, et al. (2009) Spectrophotometric evaluation of gold nanoparticles as red-ox mediator for glucose oxidase. Sens Actuat B 137:483-489.
- Sun Y, Wang HH (2007) High-performance, flexible hydrogen sensors that use carbon nanotubes decorated with palladium nanoparticles. Adv Mater 19:2818.
- Laocharoensuk R, Burdick J, Wang J (2008) Carbon-nanotuble-induced acceleration of catalytic nanomotors. Acs Nano 2:1069-1075.
- Correa-Duarte MA, Liz-Marzan LM (2006) Carbon nanotubes as templates for one-dimensional nanoparticle assemblies. J Mater Chem 16:22-25.
- Georgakilas V, Gournis D, Tzitzios V, Pasquato L, Guldi DM, et al. (2007) Decorating carbon nanotubes with metal or semiconductor nanoparticles. J. Mater Chem 17:2679-2694.
- Kong J, Chapline MG, Dai HJ (2001) Functionalized carbon nanotubes for molecular hydrogen sensors. Adv Mater 13:1384-1386.
- Wildgoose GG, Banks CE, Compton RG (2006) Metal nanopartictes and related materials supported on carbon nanotubes: Methods and applications. Small 2:182-193.
- Hanson GW, Smith P (2007) Modeling the optical interaction between a carbon nanotube and a plasmon resonant sphere. IeeeTrans Antennas Propag55:3063-3069.
- Li WZ, Liang CH, Zhou WJ, Qiu JS, Zhou ZH, et al. (2003) Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J Phys Chem B 107:6292-6299.
- Liu ZL, Lin XH, Lee JY, Zhang W, Han M, et al. (2002) Preparation and characterization of platinum-based electrocatalysts on multiwalled carbon nanotubes for proton exchange membrane fuel cells. Langmuir 18:4054-4060.
- Jiao S, Li M, Wang C, Chen D, Fang B (2007) Fabrication of Fc-SWNTs modified glassy carbon electrode for selective and sensitive determination of dopamine in the presence of AA and UA. Electrochim Acta 52:5939-5944.
- Gopalan AI, Lee K-P, Manesh KM, Santhosh P, Kim JH, et al. (2007) Electrochemical determination of dopamine and ascorbic acid at a novel gold nanoparticles distributed poly(4-aminothiophenol) modified electrode. Talanta71:1774-1781.
- Ensafi AA, Taei M, Khayamian T (2009) A differential pulse voltammetric method for simultaneous determination of ascorbic acid, dopamine, and uric acid using poly (3-(5-chloro-2-hydroxyphenylazo)-4,5-dihydroxynaphthalene-2,7-disulfonic acid) film modified glassy carbon electrode. J Electroanal Chem633:212-220.
- Li J, Zhao J, Wei X (2009) A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens Actuat B 140:663-669.
- Dhana Lakshmi AB, Michael JWhitcombe, Iva Chianella, Steven AF, SreenathSubrahmanyam EVP, et al. (2009) Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal Chem 81: 3576-3584.
- RaviShankaran D, Iimura K, Kato T (2003) Simultaneous determination of ascorbic acid and dopamine at a sol–gel composite electrode. Sens Actuators B Chem 94:73-80.
- Thiagarajan S, Tsai TH, Chen SM (2009) Easy modification of glassy carbon electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. BiosensBioelectron24:2712-2715.
- Lin X, Zhang Y, Chen W, Wu P (2007) Electrocatalytic oxidation and determination of dopamine in the presence of ascorbic acid and uric acid at a poly (p-nitrobenzenazo resorcinol) modified glassy carbon electrode. Sens Actuat B122:309-314.
- Shams E, Babaei A, Taheri AR, Kooshki M (2009) Voltammetric determination of dopamine at a zirconium phosphated silica gel modified carbon paste electrode. Bioelectrochemistry 75:83-88.
- Mazloum-Ardakani M, Beitollahi H, Ganjipour B, Naeimi H, Nejati M (2009) Electrochemical and catalytic investigations of dopamine and uric acid by modified carbon nanotube paste electrode. Bioelectrochemistry 75:1-8.
- Noroozifar M, Khorasani-Motlagh M, Parizi MB, Akbari R (2013) Highly sensitive electrochemical detection of dopamine and uric acid on a novel carbon nanotube-modified ionic liquid-nanozeolite paste electrode. Ionics 19:1317-1327.
- Suzuki I, Fukuda M, Shirakawa K, Jiko H, Gotoh M (2013) Carbon nanotube multi-electrode array chips for noninvasive real-time measurement of dopamine, action potentials, and postsynaptic potentials. BiosensBioelectron49:270-275.
- Chuang MC, Lai HY, Annie Ho JA, Chen YY (2013) Multifunctional microelectrode array (mMEA) chip for neural-electrical and neural-chemical interfaces: characterization of comb interdigitated electrode towards dopamine detection. BiosensBioelectron41:602-607.
- Kalimuthu P, John SA (2009) Electropolymerized film of functionalized thiadiazole on glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Bioelectrochemistry 77:13-18.
- Li SJ, He JZ, Zhang MJ, Zhang RX, Lv XL, et al. (2013) Electrochemical detection of dopamine using water-soluble sulfonated graphene. Electrochimica Acta 102:58-65.
- Kim DH, Oh Y, Shin H, Blaha CD, Bennet KE, et al. (2014) Investigation of the reduction process of dopamine using paired pulse voltammetry. J Electroanal Chem 717-718:157-164.
- Trefulka M, Palecek E (2009): Voltammetry of Os(VI)-modified polysaccharides at carbon electrodes. Electroanalysis 21: 1763 – 1766.
- Palecek E, Tkac J, Bertok T, Ostatna V, Palecek J (2015): Electrochemistry of non-conjugated proteins and glycoproteins. Towards sensors for biomedicine and glycomics. Chem Rev 115: 2045–2108.
- Y Lin KAW, Fallbach MJ, Ghose S, Smith JG, Delozier DM, et al. (2009) Rapid, solventless, bulk preparation of metal nanoparticle- decorated carbon nanotubes. AcsNano 3:871-884.
- Luczak T (2008) Preparation and characterization of the dopamine film electrochemically deposited on a gold template and its applications for dopamine sensing in aqueous solution. Electrochim Acta 53:5725-5731.
- Yao Y, Wen Y, Xu J, Zhang L, Duan X (2013) Application of commercial poly(3,4-ethylenedioxy-thiophene):poly(styrene sulfonate) for electrochemical sensing of dopamine. J Serb Chem Soc78:1397-1411.
- Zeng Y, Zhou Y, Kong L, Zhou T, Shi G (2013) A novel composite of SiO2-coated graphene oxide and molecularly imprinted polymers for electrochemical sensing dopamine. BiosensBioelectron45:25-33.
- Milczarek G, Ciszewski A (2004) 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane modified glassy carbon electrodes as selective and sensitive.Electroanalysis 16:1977-1983.
- Jiang X, Lin X (2005) Immobilization of DNA on carbon fiber microelectrodes by using overoxidizedpolypyrrole template for selective detection of dopamine and epinephrine in the presence of high concentrations of ascorbic acid and uric acid. Analyst 130:391-396.
- Oztekin Y, Tok M, BiliciE, Mikoliunaite L, Yazicigil Z, et al. (2012) Copper nanoparticle modified carbon electrode for determination of dopamine. Electrochim Acta 76:201-207.