Marco Picichè1*, Federico Ranocchi1, Brenno Fiorani1, Marcello Bergonzini1, Mariano Feccia1, Andrea Montalto1, Cesare D' Alessandro1, Marzia Cottini1, Riccardo Gherli1, Bruno Mariani2, Gabriella Parisi2, Giovanni Casali3, Gianpaolo Luzi4, Amedeo Pergolini5, Emilio Ferretti1, Fiorella Giacopino1, Saverio Leonardi Cattolica1, Lino Madaro1and Francesco Musumeci1
1Cardiovascular Surgery Department, San Camillo-Forlanini Hospital, Rome, Italy
2Microbiology and Virology Department, San Camillo-Forlanini Hospital, Rome, Italy
3Cardiac Surgery Department, Vito Fazzi Hospital, Lecce, Italy
4Cardiac Surgery Department, San Carlo Hospital, Potenza, Italy
5Cardiology Department, Parodi Hospital, Colleferro, Italy
*Corresponding Author:
Marco Picichè
Cardiac Surgery Department, San Camillo-Forlanini Hospital
Circonvallazione Gianicolense 87, 00152, Rome, Italy
Tel: +39 06 58701
E-mail: marco.piciche@libero.it
Received Date: February 19, 2017; Accepted Date: February 24, 2017; Published Date: February 26, 2017
Citation: Picichè M, Ranocchi F, Fiorani B, et al. Surgical Treatment of Valvular Infective Endocarditis Complicated by An Abscess: A Single Center’s Experience. Interv Cardiol J 2017, 3:1. doi: 10.21767/2471-8157.100045
Objectives: To examine the surgical treatment and mortality rate of valvular infective endocarditis complicated by an abscess in patients at a major tertiary care center.
Background: Infective endocarditis (IE) involving a heart valve is fatal if left untreated. The appearance of a comorbid abscess impacts the choice of treatment and surgical technique and, in some instances, may present unique technical challenges.
Methods: Departmental data from all patients who underwent surgery for IE at a single major tertiary care center from July 2007 to January 2016 were retrospectively screened for the presence of an intracardiac abscess. Patients with at least one confirmed abscess were examined further with respect to the surgical procedures completed and 30-day mortality rate.
Results: Over the almost nine years of data collection, we identified 14 patients (9 males, 5 females) with at least one confirmed cardiac abscess. Patients ranged in age from 28 to 77 years old (mean 57.8 ± 14 years). Various surgical procedures were performed, including aortic or/and mitral valve replacement, mitral or/and tricuspid valve repair, and a freestyle prosthetic valve implant in the pulmonary position. In two patients, surgery was extended to include the ascending aorta; while two patients underwent coronary artery bypass grafting. A patch technique was adopted whenever necessary. Overall, 12 patients survived, while one died from septic shock and another from pneumonia.
Conclusions: An abscess is a serious complication of valvular infective endocarditis that can appreciably increase the complexity of surgical intervention. In our experience, however, this seemed not to directly affect the 30-day mortality-rate, with both deaths ascribed to disseminated infection.
Keywords
Infective endocarditis; Abscess; Aortic valve; Mitral valve; Tricuspid valve; Valve repair; Surgery; Microorganisms
Introduction
Infective endocarditis (IE) is a complex condition, characterized by heterogeneity in both its presentation and course. In recent years, new microorganisms have been emerging with increasing incidence, especially affecting intracardiac devices [1]. The old term “bacterial endocarditis” has largely been replaced by the more accurate terms “infective” or “infectious” endocarditis, since infections caused by microorganisms other than bacteria are possible and not at all infrequent [2]. The reported incidence of infectious endocarditis in the general population is 3-10 episodes per 100,000 people per year, though IE is less common in the young and especially common in seniors between the ages of 70 and 80 years [3]. In most cases, IE is caused by either Streptococci or Staphylococci. The former of these two organisms is predominantly seen in native valve endocarditis (NVE) and late prosthetic valve endocarditis (PVE); while Staphylococcus aureus is more commonly found in intravenous drug abusers and in early PVE [4]. Sometimes, culture results are negative, despite the symptoms and clinical signs of IE [5]. The presence of an abscess is a complication that causes excavation of the annular tissue through an ongoing infectious process. Surgically speaking, it is more technically difficult to repair IE complicated by an abscess than standard valve replacement or repair. Herein, we review our own experience with the surgical treatment of IE that is complicated by the emergence of an intra-cardiac abscess.
Methods
Using our departmental database, clinical data were collected retrospectively on all patients treated surgically at our institution for IE between July 2007 and January 2016, a span of eight years and seven months. Over that time, a total of 74 operations for IE were performed at our institution by different surgeons. Only patients presenting with one or more intra-cardiac abscesses were included in the current analysis. Information on the surgical techniques used was collected from surgical records. Survival versus death through the first 30 days post-operatively was gleaned from hospital records.
Results
Reviewing the 74 surgical IE patients deemed eligible for screening, 14 (9 males and 5 females) were identified as having at least one cardiac abscess. These 14 patients ranged in age from 28 to 77 years old (mean=57.8). Baseline ejection fraction, measured by echocardiography, ranged from 46% to 60% (mean=54.2%). Preoperatively, in addition to their IE, four patients suffered from chronic pulmonary disease, two had creatinine levels greater than 2.0, and four were severely compromised hemodynamically.
Eight patients had already undergone one cardiac procedure. In 11 patients, only a single valve was involved, while one patient had two infected valves (aortic and mitral) and two patients had simultaneous involvement of three valves (aortic, mitral and tricuspid). In addition to standard valve replacement or repair, in four patients less standard procedures were necessary.
These included, in one patient each, removal of a pannus from a previously-implanted mitral valve; a Cabrol procedure due to involvement of the ascending aorta; extension of the anterior mitral leaflet using a pericardial patch; and a Bentall procedure complicated by the impossibility of anastomosing the right coronary ostium to the aortic prosthesis and the consequent need for right coronary artery bypass grafting with a saphenous vein graft. All of the surgical procedures performed are listed in Table 1.
| Isolated AVR |
4 |
| AVR + Pannus removal from prosthetic mitral valve |
1 |
| Bentall procedure + CABG x1 |
1 |
| Cabrol operation |
1 |
| AVR+ MV repair/replacement+ TV repair |
2 |
| AVR+MV replacement |
1 |
| Isolated MV replacement |
2 |
| Isolated MV repair (autologous patch on AML) |
1 |
| Isolated PVR (Freestyle) |
1 |
Table 1: Surgical procedures.
The main microorganisms cultured were Staphylococcus aureus and epidermidis. Corynebacterium striatum, Stenotrophomonas maltophilia, and Klebsiella pneumoniae were also found. In some cases, no microorganism was detected by blood culture. Two patients died in the Intensive Care Unit within 30 days of their operation (14.3%), one due to bilateral pulmonary infection and the other from aggressive dissemination of the infectious process resulting in severe and ultimately fatal septic shock; neither death was deemed to have directly resulted from the surgical procedure.
Discussion
Surgical treatment is required in approximately one half of patients with infective endocarditis [6]. In many cases it is preferable to administer systemic antibiotics for several weeks while monitoring echocardiographic features, to achieve reductions in the size of lesions, notably in the dimensions of vegetations. Whenever IE is accompanied by evidence of an ischemic stroke, it is generally accepted that surgery should be delayed for 2 to 4 weeks, even if the stroke is not directly attributed to the IE [7]. This delay is warranted because, in such patients, there is considerable risk that the ischemic infarct will become hemorrhagic, secondary to peri-operative heparin administration [8]. Whatever the offending microorganism is, major criteria for surgical treatment are: severely-compromised valve function; huge vegetations that are non-responsive to an appropriate course of systemic antibiotics; a high risk of embolization; uncontrolled fevers despite systemic antibiotics; and hemodynamic instability due to heart failure. Of these, the last three are generally considered to be indications for no less than urgent surgical intervention [9]. In selected cases, patients should be referred to surgery within 24 hours and this decision is made irrespective of the duration of antibiotic therapy [10].
In the already complex context of infective endocarditis, the presence of an abscess is a frightening complication. Such an abscess is defined as a paravalvular cavity, characterized by necrosis and purulent material, which does not communicate with the cardiovascular lumen (Figures 1 and 2) [6].
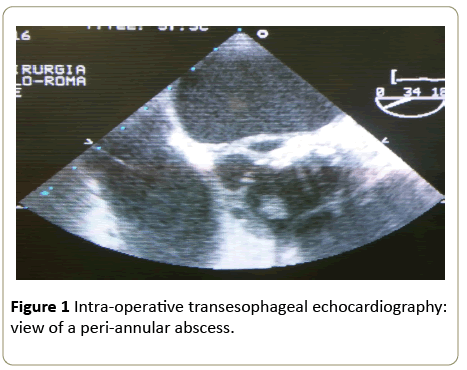
Figure 1: Intra-operative transesophageal echocardiography: view of a peri-annular abscess.
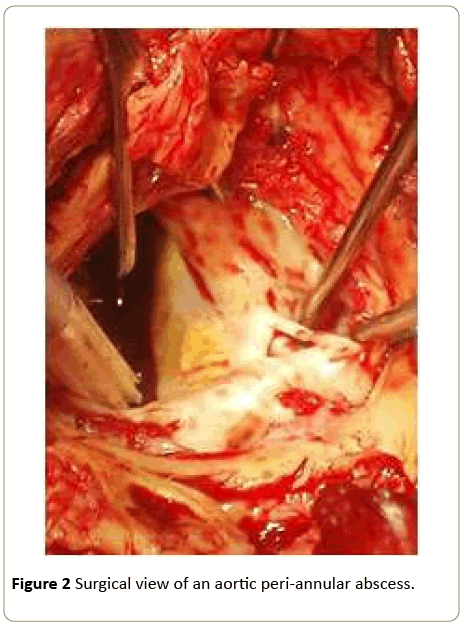
Figure 2: Surgical view of an aortic peri-annular abscess.
This scenario is frequently also complicated by a history of prior cardiac procedures, since endocarditis may attack prosthetic valves [11], as we observed in eight of the 14 patients in our series. Other typical IE lesions are vegetations the most common lesion-as well as pseudo-aneurysms, perforations, fistulas, valve aneurysms, and prosthetic heart Some patients develop arrhythmias that can further lead to hemodynamic instability [12]. Abscesses are more common in patients with prosthetic versus native valves, reported in 40% versus 19%, respectively [6]. Roughly 22% of patients with aortic valve IE have a periannular abscess, mainly due to coagulase-negative staphylococcal infections [6,13].
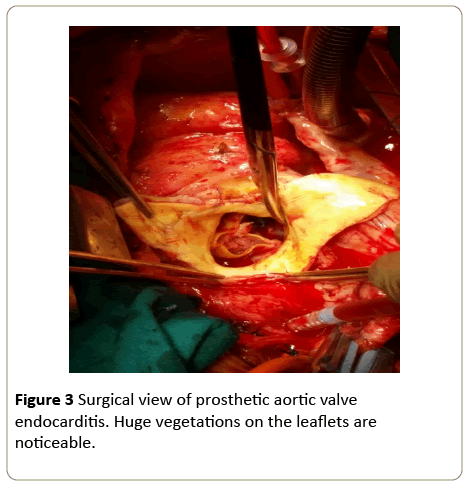
Figure 3: Surgical view of prosthetic aortic valve endocarditis. Huge vegetations on the leaflets are noticeable.
In our series, the aortic valve was more commonly associated with an abscess than any of the other valves. At surgery, it is important to achieve extensive debridement of the infected and necrotic tissues around the aortic root. In some cases, the aortic root may require reconstruction, either by replicating or patching the resected area [14]. In all of our patients with aortic valve involvement, replacing the valve with a prosthesis was necessary. Total aortic root replacement using either a biological or prosthetic composite graft may be necessary, as well as the implantation of a homograft [15]. As a general rule, we believe that when a localized abscess is no larger than a single aortic cusp, the abscess may be closed by replicating the defect between pledgeted mattress sutures, placed just below the native aortic annulus and the sewing ring of the stented prosthetic valve. When a circular abscess is larger than one aortic cusp without aortoventricular dehiscence, the defect on the aortic annulus may be reconstructed with a pericardial patch, and pledgeted sutures can be placed on this patch during AVR. When aortoventricular dehiscence (discontinuity between the aorta and the left ventricle of more than half the aorta’s circumference, after resection of all infected tissues) develops, extended aortic root replacement is indicated. For many years, the key objectives of homograft implantation have been restoring pliability in anatomically-distorted annular anatomy and reducing the risk of recurrent infection. However, the role played by homografts is currently less significant than in the past. Dr. Tirone David, whose career as a leader in cardiovascular surgery spans almost four decades, claimed that “homografts can become infected like other valves and there is no evidence that the risk of persistent or recurrent infection is different from other valves” [16]. Overall, we believe that the type of valve implanted is less important than complete debridement of all infected and edematous tissues.
As far as the mitral valve is concerned, all conventional techniques of valve replacement and repair may be used [17], employing autologous or bovine pericardium to repair leaflet perforations or annular abscesses. In our series, one patient required extension of the anterior mitral leaflet using autologous pericardium. A very challenging operation, indicated by the reconstruction of mitral-aortic continuity, is necessary if both the left fibrous trigone and central fibrous body appear completely destroyed [18-20]. This challenge can be overcome by suturing the aortic and mitral prostheses together with interposition of a Teflon strip. This should surround the mitral prosthesis for one half its circumference and the aortic prosthesis for two-thirds its circumference, eventually placing them, en bloc, in their appropriate positions [21].
Twelve of our 14 patients remained alive and were considered stable 30 days post-operatively, while one died of pneumonia and a second from sepsis. This rate of mortality (14.3%) compares favorably against those reported for two studies, both published earlier this calendar year. This includes the 24% rate of in-hospital mortality observed among 170 IE patients identified in a prospective, 17-year (1998 through 2014) population-based Italian study [22]; as well as the 20% rate of in-hospital mortality among 138 IE patients reviewed retrospectively over 16 years (1999 through 2015) at a single cardiac surgery centre [23].
Conclusion
Technical challenges due to abscesses, even in the context of redo operations, did not appreciably increase the rate of mortality in our series relative to previously-reported rates in Italy. Two of our 14 patients died, with neither death directly attributed to surgery: one due to bilateral pulmonary infection, and the other secondary to dissemination of the infection and resultant septic shock. These data suggest that the surgical treatment of IE complicated by an abscess, notwithstanding how technically demanding it can be in some cases, does not appreciably affect mortality rate in itself. In our series, both deaths were secondary to uncontrolled infection.
References
- Fernandes A, Cassandra M, Trigo J, Nascimento J, Carmo Cachulo M, et al. (2016) Cardiac device infection: Review based in the experience of a single center. Rev Port Cardiol 35: 351-358.
- Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, et al. (2016) Infective endocarditis. Nat Rev Dis Primers 2: 16059.
- Hoen B, Tornos P, Habib G (2009) Guidelines on the prevention, diagnosis and treatment of infective endocarditis (new version 2009): The Task force on the prevention and diagnosis and treatmentof infectiveendocarditis of the ESC. Eur Heart J 30: 2369-2413.
- Hoen B, Alla F, Selton Suty C (2002) Changing profile of infective endocarditis: Results of a 1-year survey in France. JAMA 288: 75-81.
- Menu E, Gouriet F, Casalta JP, Tissot-Dupont H, Vecten M, et al. (2017) Evaluation of empirical treatment for blood culture-negative endocarditis. J Antimicrob Chemother 72: 290-298.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, et al. (2015) 2015 ESC Guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC), Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36: 3075-3128.
- Jiad E, Gill SK, Krutikov M, Turner D, Parkinson MH, et al. (2017) When the heart rules the head: Ischaemic stroke and intracerebral haemorrhage complicating infective endocarditis. Pract Neurol 17: 28-34.
- Oh TH, Wang TK, Pemberton JA, Raudkivi PJ (2016) Early or late surgery for endocarditis with neurological complications. Asian Cardiovasc Thorac Ann 24: 435-440.
- Kang DH (2015) Timing of surgery in infective endocarditis.Heart 101: 1786-1791.
- Wakasa S, Matsui Y (2015) Early surgery for active infective endocarditis. Kyobu Geka 68: 586-590.
- Marcacci C, Trezzi M, Dreyfus GD (2016) Recurrent aortic prosthetic valve endocarditis: A radical additional anatomical solution. Ann Thorac Surg 102: e577-e579.
- Arai M, Nagashima K, Kato M, Akutsu N, Hayase M, et al. (2016) Complete atrioventricular block complicating mitral infective endocarditis caused by Streptococcus agalactiae. Am J Case Rep 17: 650-654.
- Molnar A, Sacui D, Manole S, Radulescu A, Beyer R (2016) The value of transthoracic and transesophageal echocardiography for the diagnosis of the native aortic infective endocarditis valve complications: A case report and literature review. Med Ultrason 18: 253-256.
- Musci M, Hübler M, Amiri A, Stein J, Kosky S, et al. (2010) Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-centre experience.Eur J Cardiothorac Surg 38: 528-538.
- Okada K, Okita Y (2013) Surgical treatment for aortic periannular abscess/pseudoaneurysm caused by infective endocarditis. Gen Thorac Cardiovasc Surg 61: 175-181.
- Cohn LH (2012) Surgical treatment of aortic valve endocarditis. In Cardiac Surgery in the Adult (4thedn), 767-773.
- Piciché M, El Khoury G, D'udekem D'akoz Y, Noirhomme P (2002) Surgical repair for degenerative and rheumatic mitral valve disease. Operative and mid-term results. J Cardiovasc Surg (Torino) 43: 327-335.
- Forteza A, Centeno J, Ospina V, Lunar IG, Sánchez V, et al. (2015) Outcomes in aortic and mitral valve replacement with intervalvular fibrous body reconstruction. Ann Thorac Surg 99: 838-845.
- Oh HK, Kim NY, Kang MW, Kang SK, Yu JH, et al. (2014) Aortic periannular abscess invading into the central fibrous body, mitral valve, and tricuspid valve.Korean J Thorac Cardiovasc Surg 47: 283-286.
- David TE, Regesta T, Gavra G, Armstrong S, Maganti MD (2007) Surgical treatment of paravalvular abscess: Long-term results. Eur J cardiothorac Surg 31: 43.
- Tedorya T, Hirota M, Ishikawa N, Omoto T (2013) Reconstruction of aorto-mitral continuity with a handmade aorto-mitral bioprosthetic valve for extensive bivalvular endocarditis. Interact Cardiovasc Thorac Surg 16: 405-407.
- Cresti A, Chiavarelli M, Scalese M, Nencioni C, Valentini S, et al. (2017) Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther 7: 27-35.
- Gatti G, Benussi B, Gripshi F, Mattia AD, Proclemer A, et al. (2017) A risk factor analysis for in-hospital mortality after surgery for infective endocarditis and a proposal of a new predictive scoring system. Infection.




