Review Article - (2017) Volume 3, Issue 1
Dimitrios Karoutsos1*, Petros Karoutsos2 and Eftychia Karoutsou3
1Department of Obstetrics and Gynecology, General Hospital of Rethymno, Athens, Greece
2Athens Medical Center, Gaia Maternity Clinic, Vas Sofias 104, Athens, Greece
3Ellinon Axiomaticon 17, Kareas, Athens, Greece
*Corresponding Author:
Eftychia Karoutsou
Ellinon Axiomaticon 17, Kareas, Athens, Greece.
Tel: 6934742976, +2130388941
Fax: 2130272713
E-mail: eutuxiakaroutsou@yahoo.gr
Received Date: December 27, 2016; Accepted Date: January 03, 2017; Published Date: January 09, 2017
Citation: Karoutsou E, Karoutsos P, Karoutsos D. The Biological Clock and Vascular Disease: Application to Pregnancy. J Clin Epigenet. 2017, 3:1. DOI: 10.21767/2472-1158.100036
Copyright: © 2017 Karoutsou E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The creation of a biological clock adjusted to way of life synchronizers influences the function of peripheral clocks and by extension, all physiological processes as well as secretion of hormones. Further, disturbance of the function of the biological clock exerts a strong impact on the pathogenesis of several disorders, including diabetes mellitus, insomnia, depression, cardiovascular disease and cancer, linking the pathophysiology of many diseases to one molecular mechanism, the 'out of tune clock'.
Keywords
Diabetes mellitus; Pathogenesis; Suprachiasmatic nucleus; Cryptochrome
What is the biological clock?
In mammals, the components of the biological clock function peripherally in a complex network and modulate the transcription of specific target genes and their products, expressed rhythmically in a 24 h rhythm. The central biological clock includes the suprachiasmatic nucleus, which rhythmically 'tunes' peripheral clocks (Figure 1) [1-8].
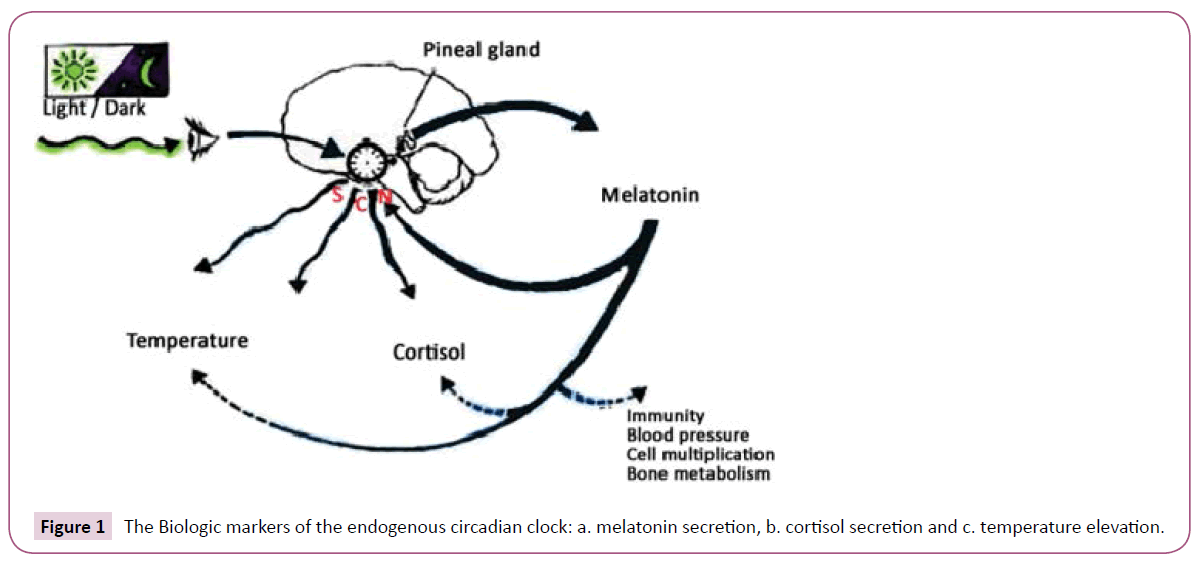
Figure 1: The Biologic markers of the endogenous circadian clock: a. melatonin secretion, b. cortisol secretion and c. temperature elevation.
Having the molecular basis of SCN being clarified the so-called core clock genes such as per1-2, cry1-2, bmal1 [9,10] and the demonstration that these genes are rhythmically expressed in peripheral tissues, including the liver, adrenal and pituitary glands [11-13], this central clock model is reshaped [14-16].
Clock/Bmal, formed by the product of the genes circadian locomotor output cycles kaput [Clock] and brain and muscle aryl hydrocarbon receptor nuclear translocator like-Arntl (Bmal) represents the transcription initiator of the feedback loops [17].
Clock/Bmal binds to E-box in the promoter regions of target genes Period homolog 1, 2 and 3 genes (Per1, Per2, Per3), Cryptochrome genes (Cry1, Cry2), retinoic acid-related orphan receptor (Rora, Rorb, Rorc) and Rev-Erb nuclear orphan receptor(Rev-Erbα, Rev- Erbβ) to activate their expression [18,19].
Transcription of Pers and Crys is initiated during the day, while degradation during the night. PER and CRY proteins enter the nucleus, probably as a multimeric complex (PER/CRY), and inhibit Clock/Bmal-mediated transcription [20,21].
As to the role of the PER/CRY complex, Cryptochrome (CRY) is a blue-light sensor, which regulates neuronal firing rate, that is circadian entrainment in Drosophila melanogaster [22]. Light activates CRY. When CRY is activated TIM [timeless] degrades, tuning the clock daily. In the absence of cry, the clock can still be activated by light, though the mechanisms are unclear; possibly, there are two types of clock neurons having differential sensitivities to light and temperature [23].
With regard to the remainder feedback loop, the ROR/Bmal/Rev- Erb (RBR) frames the original shape [20]. ROR acts as an activator of Bmal and Rev-Erb as an inhibitor which results in tuning of Bmal transcription [24,25]. Suppression of Rev-Erbα expression resulted in elevated Clock mRNA expression consistent with Rev- Erbα's role as a transcriptional repressor, adding a regulation role of the activity of the Bmal1/Clock heterodimer by regulation of the expression of both the Bmal1 and Clock genes directly (Figure 2) [26]. Other nuclear receptor family members, such as peroxisome proliferator activated receptors-α/γ, estrogen receptor-α, and retinoic acid receptor through their ligands link the clock mechanism with peripheral stimuli output [27].
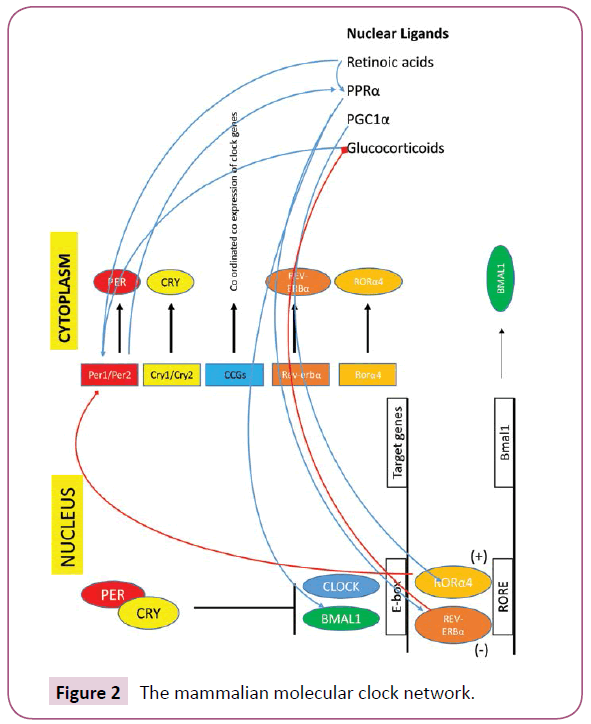
Figure 2: The mammalian molecular clock network.
Circadian entrainment and metabolism
Secondary peripheral clocks are found in various tissues, such as the liver, pancreas, and adipose tissue. These clocks control the rhythmic patterns of metabolic processes. If the function of the central or peripheral clocks is disturbed diabetes mellitus or obesity emerges [28]. For instance, Lamia et al. [29] have described the metabolic phenotype of total vs. liver-specific Bmal1-deficient mice. The latter present with hypoglycemia, in the presence of an intact central biological clock mechanism, emphasizing the importance of the function of peripheral clocks for the homeostasis of glucose.
Circadian Rhythms and Cardiovascular Disease
Circadian expression of clock genes was reported in cardiovascular tissues by several investigators. The measurement of core clock gene expression revealed rhythmicity in the heart, aorta, and kidney [30,31]. Almost all of the known core clock genes, such as clock, bmal1, cry1/2, and per1/2/3 genes, are expressed in the heart [32]. Expression of some genes in the heart oscillates with circadian rhythmicity. Regulation of heart's systolic function, oxygen metabolism and resistance to ischaemic disorders is done by the clock seated in the cardiomyocyte. These same biological processes are also directly influenced i.e., by modification of proteins by monosaccharides of O−linked β− N−acetylglucosamine (O−GlcNAc) [33]. Despite of the integral relation between clocks and metabolism, little is known regarding the direct influence of a peripheral clock on cellular responses to fatty acids. Tsai et al. [34] to address this issue utilized a genetic model of disrupted clock function specifically in cardiomyocytes in vivo (termed cardiomyocyte clock mutant). There are data that the cardiomyocyte clock modulates triglyceride metabolism in the myocardiocyte [34].
Further, Rev-Erbα is present in vascular wall cells including macrophages [35-37]. Rev-Erbα downregulates toll like receptor, thus inhibiting the inflammatory cascade. These data suggest a positive impact of Rev-Erbα on atherosclerotic lesions. Likewise, Rev-Erbα has been identified as a potent negative regulator of plasminogen activator inhibitor (PAI)-1, an important inhibitor of the fibrinolysis cascade that may promote the development of atherothrombosis [38]. In vitro results suggest a regulatory link between Rev-Erbα and the NF−kappaB pathway in vascular wall cells, too [37]. Rev-Erbα, besides exerting a regulatory role on lipid metabolism, adipogenesis and vascular inflammation, also cross talks with several other nuclear receptors involved in energy homeostasis. Therefore this nuclear orphan receptor by coupling metabolic and circadian signals [38,39], gives a time cue to metabolism−becoming so a potential modulator of the morning susceptibility to myocardial infarction [40].
The transcript levels of potassium channels Kv1.5 and Kv4.2 also indicate apparent circadian fluctuation [41]. The circadian variation of the onset of cardiac arrhythmia might be attributed to intrinsic circadian rhythms in potassium channel expression. Although the exact mechanisms regulating the circadian expression of these genes are unknown, some of these genes may be clock output genes in the heart. In the heart with hypertrophy in rat’s experiments [42], the tuned transcription of anp is inhibited along with hlf and dbp. This reduction of circadian expression of anp may perturb the adaptive response of cardiomyocytes to external stimuli and accelerate the hypertrophic processes. The circadian rhythm of mean blood pressure [MBP] was selectively disrupted in sinoaortic denervated rats under 12 h light−dark cycles as a result of an increase in MBP during the light period and disappeared under constant darkness [43].
Regarding the clinical setting, Manios et al. [44] recently evaluated the association between the rate of blood pressure variation, derived from ambulatory blood pressure monitoring data analysis, and the severity and topography of coronary artery lesion in a cohort of normotensive patients with suspected coronary artery disease. Abrupt variations in blood pressure are related to atherosclerosis and enhance cardiac hypertrophy [45].
A group from Amsterdam had already determined the continuous 24 h profile of mean arterial pressure, heart rate, stroke volume, cardiac output, and total peripheral resistance in eight healthy ambulatory volunteers. During the night time, compared with the daytime average, there was a decrease in blood pressure (9 mm Hg), heart rate (18 beats per minute), and cardiac output (29%); stroke volume showed a small decrease [7%], and total peripheral resistance increased unexpectedly by 22% [46].
Kollias et al. [47] earlier demonstrated a circadian variation of endothelial function and arterial stiffness in hypertension, that is endothelial function is decreased in the early morning in hypertensive patients, whereas arterial stiffness is increased in the evening. No ulterior, Zakopoulos et al. [48] presented that diurnal variations of continuously monitored interstitial glucose concentrations significantly associate with blood pressure levels in both normotensive and hypertensive humans, indicating a common pathway of circadian autoregulation, probably stemming from both central mechanisms and peripheral inputs which in turn exert a feedback loop.
Actually, the amplitude of this matter disaggregated by Guo et al., in American Heart Journal in 2003. Results of these studies confirm that most cardiovascular physiological parameters (such as heart rate, blood pressure, electrocardiogram indices) and pathophysiological events [myocardial ischemia/infarction, sudden cardiac death) show circadian rhythms [49]. Interestingly, Mann et al. had prior showed that patients with primary autonomic failure and very low sympathetic and parasympathetic activities also have a high incidence of nondipping [50], suggesting that it is the inability to modulate autonomic tone that is responsible for the nondipping phenomenon [51]. Other factors be taken into account, a quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure clearly indicated there was no significant diurnal variation of BP which is independent of activity [52]. To a deeper approach, in normoalbuminuric normotensive type 1 diabetic patients without any degree of autonomic dysfunction, according to traditional cardiovascular tests, diastolic BP night/day ratio is associated with an increased glomerular filtration rate and an increased extracellular volume. The disruption of the circadian rhythm of sympathovagal activity in non−dipper patients was associated with higher levels in systolic and diastolic blood pressure and with a reduced decline in their levels during the night [53]. Dippers suffering from hypertension independently present with cardiovascular events without having a positive history [54].
Is there a clinical relevance of the biologic clock to pregnancy? The endogenous placental clock: Circadian variation of the HPA axis is dependent on clock gene rhythms in the hypothalamus, but it is not known whether pregnancy-induced changes in maternal glucocorticoid levels are mediated via this central clock [55]. Does a placenta function like a tuned clock? Recent recognition that clock genes are expressed in the placenta, together with observations linking circadian disruption with compromised placental function, suggests that circadian variation may be an important component of the normal placental phenotype [56]. Of note is that, for both pregnant women with complications in either the second or third trimester and healthy pregnant women, melatonin levels were the highest at night, decreased throughout the early morning, and were the lowest during the day, demonstrating significant daily fluctuations [57].
In fact, maternal melatonin enters the fetal circulation with ease providing photoperiodic information to the fetus. Melatonin protects placenta from maternal oxidative stress, modulates endocrine function and tunes the function of immunocompetent cells [58]. Melatonin reduces oxidative damage in the placenta and may improve hemodynamics and nutrient transfer at the placental-uterine interface [59].
Pérez et al. has shown the existence of a biological clock in this tissue, by studying the expression of circadian locomotors output cycles kaput [Clock], brain and muscle arnt-like (Bmal)1, period(Per)2, and cryptochrome (Cry)1 mRNAs at 00, 04, 08, 12, 16, and 20 h by qPCR. The clock genes are present in human placenta, reinforcing the hypothesis of the placental biological clock [60]. Moreover, alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock determine the length of parturition [61].
Is a Dysfunction of the Placental Clock Related to Preeclampsia?
Maternal oxidative stress predisposes to preeclampsia. Simultaneously, melatonin, as a seasonal factor, has been suggested to be involved in a successful pregnancy [62-64]. Το be brought to safe conclusions we should carefully consider the pathophysiologic process taking place in preeclampsia (Figure 3). Κarteris showed that placental CRH-R1 alpha mRNA levels (as shown by quantitative RT-PCR) and protein levels [shown by Western blotting analysis] were significantly reduced in all of the complicated pregnancies [65]. Previously, Karteris working team supported that there is a CRH placental clock that is active from the early stages of pregnancy and determines the length of gestation and the timing of parturition. CRH can influence human reproductive tissue function via specific CRH receptors. Two distinct CRH receptors have been cloned (R1 and R2) that exist as two alternatively spliced forms (alpha and beta) [66]. Actually, CRH participates in the pathophysiology of pre-eclampsia through the induction of macrophage-mediated apoptosis of extravillous trophoblasts (Figure 4).
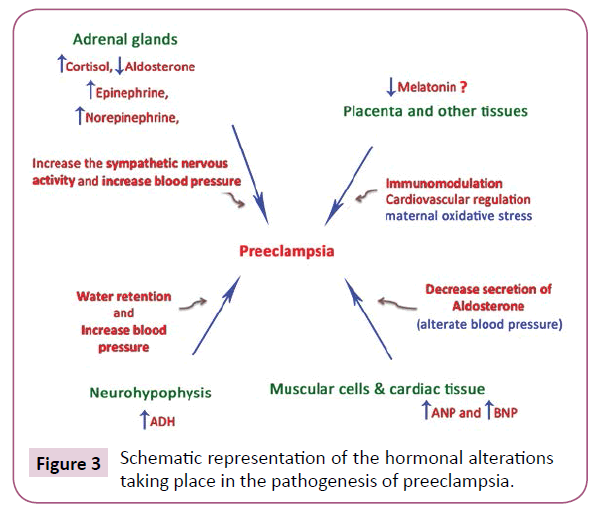
Figure 3: Schematic representation of the hormonal alterations taking place in the pathogenesis of preeclampsia.
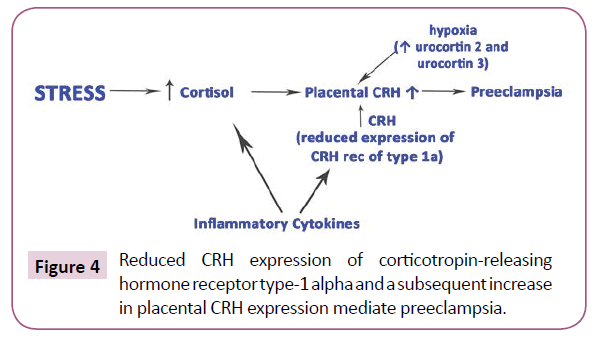
Figure 4: Reduced CRH expression of corticotropin-releasing hormone receptor type-1 alpha and a subsequent increase in placental CRH expression mediate preeclampsia.
It was found that the expression of CRH was increased in trophoblasts of the placental bed biopsy specimens from preeclamptic pregnancies [67]. Nevertheless, a question mark remains as to the role of melatonin in the pathophysiology of preeclampsia and the associated input driven by the biological clock.
Shimada showed that pregnant women with hypertension or diabetes had lower levels of salivary melatonin during the day than healthy pregnant women [57]. Moreover, Reiter suggested that due to the potent antioxidant actions of melatonin, coupled with its virtual absence of toxicity, this indoleamine may have utility in the treatment of pre-eclampsia, intrauterine growth restriction, placental and fetal ischemia/reperfusion [68].
Clinical approach of vascular disease including the time of pregnancy
Physicians, care managers, and patients agreed regarding the positive impact on patient health and self-management, and attributed the outcomes to the strong “partnership” between the care manager and the patient and the collaboration between the physician and the care manager [69]. Previously, it was confirmed by Ciccone et al. that carotid atherosclerosis is associated with coronary atherosclerosis, highlighting the importance of screening for ischemic heart disease in patients with asymptomatic carotid plaques, considering eventually plaque morphology[symmetry, composition, eccentricity or concentricity of the plaque] for patient stratification [70]. Moreover, considering the disturbed function of the placental clock and preterm delivery, different functional cardiac characteristics have been observed in term and preterm neonates by echocardiography and tissue doppler imaging, while increased size of the left ventricle and altered left ventricular diastolic function was found in children born very low birth weight preterm [71,72]. What do we observe? Certainly, atherosclerosis and ischemia are common manifestations of a disorder, which at least initially involves unwound clock function. Further, cardiovascular disease of the mother or reasons such as lower placental perfusion, increased blood pressure in the mother and preeclampsia, often resulting in intrauterine growth restriction (IUGR), perpetuate vascular disease. That is because developmental cardiovascular alterations and potential mechanisms link preterm birth to the risk of hypertension and cardiovascular diseases into adulthood [73]. Of note is that, impairments to vascular system development may initially occur before preterm birth via IUGR and exposure to an inflammatory and antiangiogenic environment [74].
Conclusion
Given the broaden role of peripheral circadian loops in lipid metabolism affecting the development of atherosclerosis and vascular wall pathophysiology or autonomic tone and blood pressure per se, regulation of the expression of both the Bmal1 and Clock genes represents a promising target for the treatment of cardiovascular abnormalities resulting from chronic aberrant circadian rhythms. Perhaps, treatment with melatonin is the appropriate therapy for preeclampsia.