Keywords
SIV nef deletion; Live attenuated virus; Viral kinetics;
Intradermal inoculation.
Introduction
A live attenuated form of SIVmac239 containing a deletion in
the nef gene has been used to study the role of nef in
lentivirus viral infectivity and disease progression [1, 2]. The
nef gene is expressed early in SIV infection to promote the
replication of the viral genome [3-7] and in maintaining viral
load in vivo [8]. The observed enhancement of viral infectivity
was independent of cell line used, multiplicity of infection and
number of virus replication cycles [9]. Superior growth and
infectivity was observed in both human and macaque primary
cell cultures infected with SIV and with recombinant HIV
expressing SIV nef [10, 11]. The mechanism by which SIV Nef
enhances viral infectivity is not completely understood,
however, Nef has been shown to down regulate CD4 and
MHC1-1 expression, as well as interfere with CD8 T-cell
response in monkeys [12]. Additional functions attributed to
Nef include interfering with the endocytosis machinery,
directing the activation status of infected cells and disrupting
the actin cytoskeleton to render cells more permissive to viral
infection [13]. Based on the many roles of Nef, together with
the findings that nef-deficient mutants produce much lower
viral loads accompanied by decreased pathogenicity,
demonstrates that nef is a key factor in viral fitness and
suggest that nef-deficient SIV can act as a live attenuated virus
vaccine [8].
Traditionally, to elucidate the characteristics of a live
attenuated SIV virus, it is inoculated intravenously into SIV
naïve monkeys [14-16]. Most animals develop detectable
viremia a few weeks post-inoculation [14, 16] and an immune
response to the attenuated virus is often seen during this
viremia [14, 17]. Although infection of rhesus macaques by
intravenous route are used to study viral pathogenesis and disease progression, additional routes of virus inoculation
should be established in this model to fully understand the
features of a live attenuated virus.
This paper describes the effect of intravenous inoculations
of SIVΔnef virus into monkeys infected with SIVmac239. We will
also discuss the ability of SIVΔnef to infect rhesus macaques
through an intradermal route.
Materials and Methods
Animals
Rhesus macaques from the SIV-naïve colony at California
National Primate Research Centre (CNPRC) were selected
based on age range of 4 to 7 years and body weight range of
6-10 kg, with size as uniform as possible within genders. The
animals were pair-caged in a BSL 2+ facility during the study
period. Body weight and temperature was recorded regularly.
Blood samples were collected for clinical haematology, clinical
chemistry, viral and immunological assays. Urine samples were
collected by cystocentesis for urinalysis.
All animals were housed at the California National Primate
Research Centre (CNPRC) in accordance with American
Association for Accreditation of Laboratory Animal Care
Standards and the "Guide for the Care and Use of Laboratory
Animals," by the Committee on Care and Use of Laboratory
Animals of the Institute of Laboratory Resources, National
Resource Council [18].
The study was approved by the Institutional Animal Care
and Use Committee of the University of California Davis.
Determination of host genetics
MHC typing for 2nd class I alleles (Mamu-A*01, A*08, B*01,
B*08, B*17, B*29) was performed by the Veterinary Genetic
Laboratory (University of California, Davis) and the AIDS
Vaccine Research Laboratory (University of Miami) using
methods previously described [19-21]. Animals were assigned
to have random distribution of MHC alleles and comparable
virus levels at week 4 pi.
Infection with SIVmac239
SIVmac239 stock (batch number 2011) was propagated on
rhesus PBMC and had a titer of 50,000 TCID50 per ml. All
animals were injected with SIVmac239 by intravenous route
with 100 TCID50 at time 0.
Virus preparation and inoculation of SIVΔnef
by intravenous route
nef-deleted SIVmac239 plasmid was obtained from NIH AIDS
Reagent Program (catalog #12246), and virus stocks were
produced in CEMX174 cells. The nef deletion was confirmed by
sequencing and viral stock with titre of 3.2 × 106 TCID50 per ml
was selected for macaque infection studies. At 8 and 10 weeks
pi, animals were injected with either High Dose SIVΔnef (4×106 TCID50), Low Dose SIVΔnef (4 × 105 TCID50), or Placebo
consisting of RPMI 1640 medium.
Inoculation of SIVΔnef by intradermal route
Two animals were inoculated intradermally with 0.8 ml of
3.6 million TCID50 per ml. Due to the limited volume that can
be administered intradermally, animals were inoculated at 8
injection sites of 0.1 ml each; thus, the total dose administered
was approximately 3 million TCID50.
Clinical monitoring and sample collection
Routine daily observations were performed according to
CNPRC SOP # F01: “Morning Health Check of Indoor Animals”.
A subjective, non-quantitative observation of food
consumption was made as part of the animal husbandry
monitoring. Physical exams, including measurement of body
weight and temperatures were performed at every study
period. At specified study dates, the sedation and physical
exams were combined with the collection of samples (blood
and urine) for viral load determination, haematology, clinical
chemistry, flow cytometry, cryopreservation of serum, and
urinalysis.
Clinical laboratory analysis
Serum was tested for a standard clinical chemistry panel.
Components measured include sodium, potassium, chloride,
total carbon dioxide (TCO2), inorganic phosphorous, calcium,
BUN (blood-urea-nitrogen), creatinine, glucose, total protein,
albumin, alanine aminotransferase (ALT), aspartate
aminotransferase (AST), creatine phosphokinase (CPK),
alkaline phosphatase, gamma glutamyl transferase (GGT),
lactate dehydrogenase, triglycerides, cholesterol, total and
direct bilirubin.
A sample of whole blood were collected for red blood cell
(erythrocyte) count, white blood cell (differential) count,
haemoglobin, haematocrit, mean corpuscular volume, mean
corpuscular haemoglobin, mean corpuscular haemoglobin
concentration, platelet counts, plasma protein and fibrinogen.
Urine was collected via cystocentesis according to standard
operating procedures. It was analysed by Clinical Laboratory
staff at CNPRC for specific gravity by a refractometer; the pH
and the presence of protein, glucose, ketone, bilirubin, and
occult blood was determined using Multistix 10 SG strips
(Siemens Healthcare Diagnostics). Urine sediments were
examined microscopically.
Plasma viral load
SIV RNA levels in the plasma samples were tested by RT-PCR
assay for SIV gag, as previously described [22]. The remaining
blood was subjected to density gradient (Lymphocyte
Separation Medium) centrifugation to isolate peripheral blood
mononuclear cells (PBMC). Statistical analyses were
performed using 1-way ANOVA for viral loads at week 4. A
value of P of <0.05 was considered statistically significant.
Phenotyping of lymphocyte populations
An aliquot of EDTA-anti-coagulated blood was combined in
a single tube with the following antibodies, all from BD
Biosciences (San Jose, CA): Anti-CD3-Pac-blue (clone SP34-2),
anti-CD4-PerCP-Cy5.5 (clone L200), anti-CD8-VK500 (clone
SK1), anti-CD14-FITC (clone M5E2), anti-CD16-PE-Cy7 (clone
3G8), anti-CD20-APC (clone L27) and anti-CD28-PE (clone
L293). Multi-parameter flow cytometric analysis was used to
test for the expression of CD3, CD4, CD8, CD14, CD16 and
CD20 to determine the percentage of total T cells (CD3+),
helper T cells (CD4+), cytotoxic T cells (CD8+), monocytes
(CD14+), NK cells (CD16+) and B cells (CD20+).
Criteria for euthanasia and necropsy
Euthanasia of animals with simian AIDS was determined by
established criteria of one or more of the following clinical
observations indicative of a severe life-threatening situation:
weight loss of >15% in 2 weeks or >25% over any time course;
chronic diarrhoea or other opportunistic infections
unresponsive to treatment; inability to maintain body heat or
fluids without supplementation; obtundation; neurologic
deficits; persistent, marked hematologic abnormalities,
including anaemia (<20%), thrombocytopenia with petiachiae
or ecchymosis, and hypoproteinemia with edema.
Results and Discussion
Inoculation of SIVΔnef virus in SIV infected
rhesus macaque
Eighteen rhesus macaques infected with SIVmac239 at day 0
were assigned to three study groups, Group 1 Placebo, Group
2 Low Dose SIVΔnef, and Group 3 High Dose SIVΔnef (Table 1)
based random distribution of MHC alleles and comparable
viral loads at week 4 pi. Twelve out of eighteen animals tested
positive for MHC alleles A08, B01, B08, B17 and B29 is shown
in Table 2. None of the animals were positive for the
protective allele A01. To randomly distribute the MHC alleles,
a value of +1 was given to protective alleles (A01, B08, B17), -1
to negative alleles (B01) and 0 to neutral alleles (A08, B29).
The total MHC score for female rhesus macaque in the
Placebo, Low, and High Dose group were 0, 0 and -1,
respectively. The total MHC score for male animals in those
groups were 0, 0, and +1. Viral loads in SIV infected rhesus
macaques were also compared at 4 weeks pi. Mean viral load
(Log 10) in female animals were 5.26, 5.26 and 5.32 (p=0.97)
for Placebo, Low and High Dose groups. For male rhesus
macaques, mean viral loads in those groups were 5.41, 5.33
and 5.18 (p=0.88). The viral loads in all animals were
comparable regardless of MHC alleles at 4 weeks pi. This
suggests that these genotypes did not influence the acutephase
plasma virus concentrations.
| Groups |
M |
F |
SIVmac239
Week 0
IV route |
Study Agent
Week 8 and 10
IV route |
Total Study PhaseDuration |
| Placebo |
3 |
3 |
100 TCID50 units |
Placebo |
16 weeks |
| Low Dose |
3 |
3 |
100 TCID50 units |
ΔnefSIVmac239, 4e5 TCID50 |
16 weeks |
| High Dose |
3 |
3 |
100 TCID50 units |
ΔnefSIVmac239, 4e6 TCID50 |
16 weeks |
Table 1: Overall experimental design.
| Group |
Animal ID |
Sex |
MHC typing |
Log of viral RNA/ml plasma
(week 4) |
| A01 |
A08 |
B01 |
B08 |
B17 |
B29 |
MHC Score* |
| Placebo |
38890 |
F |
- |
- |
+ |
- |
+ |
+ |
0 |
4.93 |
| Placebo |
38902 |
F |
- |
- |
- |
- |
- |
- |
0 |
5.3 |
| Placebo |
38924 |
F |
- |
- |
- |
- |
- |
- |
0 |
5.56 |
| Low |
39139 |
F |
- |
- |
- |
- |
- |
- |
0 |
5.36 |
| Low |
39204 |
F |
- |
- |
+ |
- |
+ |
+ |
0 |
4.94 |
| Low |
39600 |
F |
- |
+ |
- |
- |
- |
- |
0 |
5.48 |
| High |
39756 |
F |
- |
- |
+ |
- |
- |
- |
-1 |
4.79 |
| High |
39944 |
F |
- |
+ |
- |
- |
+ |
+ |
1 |
5.59 |
| High |
40184 |
F |
- |
+ |
+ |
- |
- |
- |
-1 |
5.58 |
| Placebo |
38092 |
M |
- |
- |
+ |
- |
- |
- |
-1 |
5.4 |
| Placebo |
39330 |
M |
- |
- |
- |
- |
+ |
- |
1 |
5.56 |
| Placebo |
40233 |
M |
- |
- |
- |
- |
- |
- |
0 |
5.28 |
| Low |
39473 |
M |
- |
+ |
- |
- |
- |
- |
0 |
5.45 |
| Low |
39616 |
M |
- |
- |
- |
- |
+ |
+ |
1 |
5.11 |
| Low |
40358 |
M |
- |
- |
+ |
- |
- |
- |
-1 |
5.43 |
| High |
39863 |
M |
- |
- |
- |
- |
- |
- |
0 |
5.36 |
| High |
40062 |
M |
- |
- |
- |
- |
- |
- |
0 |
6.04 |
| High |
40124 |
M |
- |
+ |
- |
+ |
- |
- |
1 |
4.15 |
Table 2: MHC typing and distribution of animals into Placebo,
low and high dose group.
At 8 and 10 weeks pi, animals were inoculated intravenously
with either RPMI media, 4 × 105 TCID50 or one-log fold higher
dose 4 × 106 TCID50 of the attenuated virus. Animals were
monitored for changes including clinical haematology, clinical
chemistry, plasma viral loads and lymphocyte cell counts.
In all animals, weights and temperatures were collected
regularly throughout the study duration. No significant
differences in temperature were observed and the weight of
most animals remained close to pre-infection values or
increased (data not shown).
In other clinical observations, animal 40358 from the Low
Dose group had two reports of mild dermatitis prior to
SIVmac239 infection 4 months apart. After inoculation with
SIVmac239, moderate dermatitis was observed at 4 weeks, and
topical treatments were applied starting at 12 weeks pi.
Animal 38890 from the Placebo group had intermittent reports
of mild dermatitis throughout the study, but no special
treatments were given.
Plasma viral loads in SIV-infected rhesus macaques did not
change after two intravenous inoculations with low or high
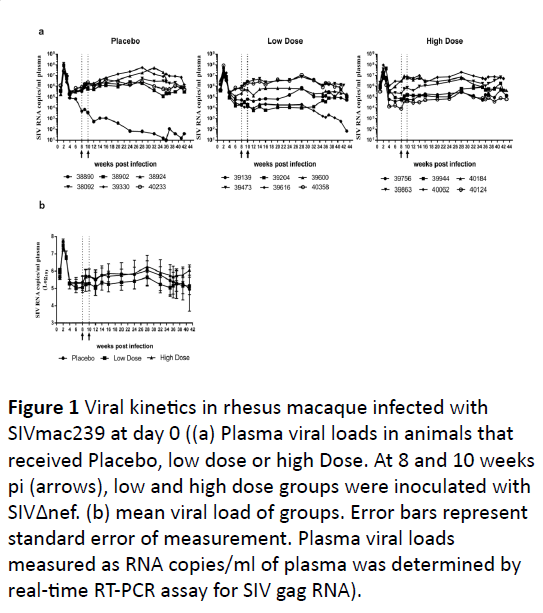
dose of SIVΔnef compared to Placebo control (Figure 1a). One
animal in the Placebo group, 38890 had progressively low viral
loads over time with a 3 log reduction in viral load by 16 weeks
pi.
This animal was positive for Mamu-B17 and Mamu-B29
allele, which has been demonstrated to show a degree of viral
control after infection with pathogenic SIVmac239 [23]. Animals
39204, 39944 and 39616 was also positive this allele but did
not demonstrate a reduction in viral loads.
This is consistent with previous report, in which only 26% of
B17 and B29 positive rhesus macaques infected with
SIVmac239 showed elite control of virus [23]. No differences
were seen in plasma viremia between placebo or SIVΔnef
inoculated animals (Figure 1b), which is consistent with
historical data of SIVmac239 infection [24-26].
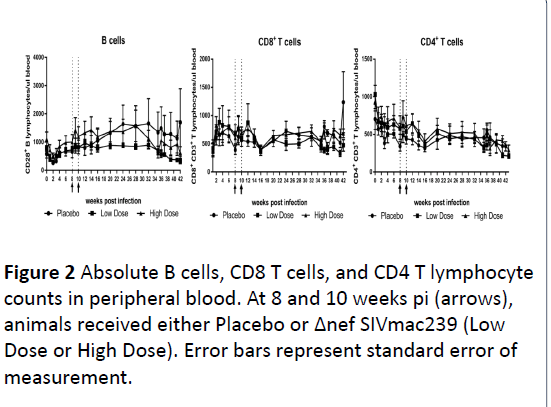
Acute infection with SIVmac239 was characterized by a
marked decline in B cells in the peripheral blood in Placebo,
Low, and High Dose groups with 47.6+21.2%, 60.1%+28.5%
and 49.5%+11.4% decline, respectively, at 2 weeks pi
compared to baseline levels. However, there was a rapid
recovery of peripheral B cells with restoration to pre-infection
levels by 4 to 6 weeks pi (Figure 2).
Figure 1: Viral kinetics in rhesus macaque infected with
SIVmac239 at day 0 ((a) Plasma viral loads in animals that
received Placebo, low dose or high Dose. At 8 and 10 weeks
pi (arrows), low and high dose groups were inoculated with
SIVΔnef. (b) mean viral load of groups. Error bars represent
standard error of measurement. Plasma viral loads
measured as RNA copies/ml of plasma was determined by
real-time RT-PCR assay for SIV gag RNA).
Total T lymphocyte counts remained steady during the
course of the infection, including steady state CD8 T
lymphocytes. In addition, there were no changes in NK and
monocyte levels. A decline in peripheral blood CD4 T
lymphocyte was observed in during the first 8 weeks pi,
followed by a gradual decline up to 43 weeks pi in all groups.
CD4 T lymphocyte decline is an important marker of disease
progression, and is consistently observed in SIVmac239 rhesus
macaque models for AIDS [27, 28].
Figure 2:Absolute B cells, CD8 T cells, and CD4 T lymphocyte counts in peripheral blood. At 8 and 10 weeks pi (arrows),
animals received either Placebo or Δnef SIVmac239 (Low Dose or High Dose). Error bars represent standard error of
measurement.
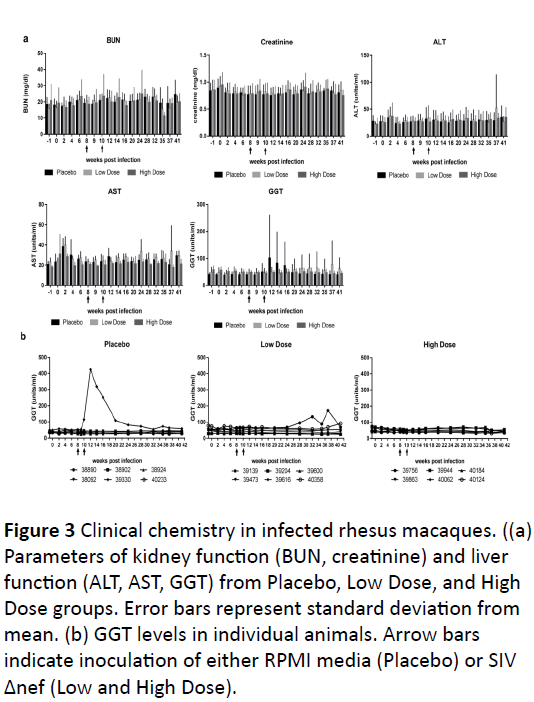
Several parameters indicative of kidney function (BUN,
creatine) and liver function (ALT, AST, GGT) and immune
system (WBC), are shown in (Figure 3a).
In most animals, the ranges were within the normal values
for clinical haematology, chemistry, and urinalysis tests as
described in the methods section. In one animal from the
Placebo group, 39300 developed increased levels of GGT,
alkaline phosphatase and C-reactive protein from week 10
onwards.
Animal 39139 inoculated with low dose SIVΔnef also had
increased levels of GGT starting a 20 weeks pi (Figure 3b). This
is indicative of hepatobiliary disease, which is a common
complication of SIV infection.
Figure 3:Clinical chemistry in infected rhesus macaques. ((a) Parameters of kidney function (BUN, creatinine) and liver
function (ALT, AST, GGT) from Placebo, Low Dose, and High Dose groups. Error bars represent standard deviation from
mean. (b) GGT levels in individual animals. Arrow bars indicate inoculation of either RPMI media (Placebo) or SIV Δnef (Low and High Dose).
Study animals were continually monitored during chronic
phase of infection until the progression of simian AIDS in some
animals. Placebo animal 38924 showed signs of terminal SIV
infection and was necropsied by 36 weeks pi. The animal had a
clinical history of diarrhoea and gradual weight loss of 25%
compared to pre-infection weight. Two other animals met the
criteria for euthanization at week 41. Animal 40358 from the
Low Dose group had reduced appetite and developed
respiratory distress and animal 39863 from the High Dose
group showed severe anaemia and icterus. The study was
concluded by 43 weeks post infection, and all animals had
signs of advanced SIV infection including lymphadenopathy
and spleenopathy.
The inoculation of SIVΔnef during the early chronic phase of
infection in the Low and High Dose groups did not influence the progression of disease in these animals compared to
Placebo. Inoculation of SIVΔnef did not change viral loads in
SIV wild type-infected rhesus macaques compared to Placebo
group. CD4 counts also steadily declined and clinical symptoms
were absent or mild. The lack of changes suggest that
inoculation of SIVΔnef at 8 and 10 weeks post infection did not
modify or accelerate the outcome of wild type infection.
Infection with SIVΔnef virus by intradermal
route
Two animals (39423 and 40307) were inoculated
intradermally with SIVΔnef. Both animals were viremic starting
one week after inoculation (Figure 4a), with peak levels at 1 or
2 weeks (130,000 to 440,000 SIV RNA copies/ml). By 10 weeks
pi, plasma viremia declined to below the limit of detection
(<30 copies/ml; animal 39423) to 3,700 copies/ml (animal
40307).
Figure 4:Plasma viremia of 2 animals inoculated intradermally with SIVΔnef (a) SIV RNA copies/ml in animals
39423 and 40307 for up to 10 weeks pi. The limit of detection for this assay is 30 copies/ml. (b) Comparison of plasma viremia of SIVΔnef with wild type SIVmac239.
These plasma levels are indistinguishable from those
described previously in the literature for animals infected
intravenously with SIVΔnef, namely peak levels of ~ 104 to 105 SIV RNA copies/ml and viral set point at week 10 ranging from
undetectable to ~ 103 SIV RNA copies/ml plasma [16, 29]. In
accordance with these moderate virus levels, animals did not
exhibit any clinical signs during the observation period. In
comparison to wild type SIVmac239, the two animals infected
with the attenuated strain had a two log decrease in plasma
viral load (Figure 4b). This finding confirms the infectivity of
SIVΔnef by an intradermal route.
In conclusion, the study demonstrates the attenuated
phenotype of SIVΔnef virus and further understanding of
SIVΔnef virus may lead to vaccine constructs that can perhaps
alter wild type SIV viral infectivity and disease progression.
Acknowledgement
We thank Linda Hirst, Joyce Lee, Colony Services, and
Pathology, Veterinary, and Clinical Laboratory staff of the
California National Primate Research Centre for expert
technical assistance. We thank Teresa M Giret and David
Watkins of the AIDS Vaccine Research Laboratory, University of Miami Miller School of Medicine for the MHC typing; Leidos
Biomedical Research, Inc., Frederick National Laboratory,
Frederick, Maryland 21702 for the viral RNA and DNA load
determinations.
References
- Gibbs JS, Regier DA, Desrosiers RC (1994) Construction and in vitro properties of SIVmac mutants with deletions in "non-essential" genes. AIDS Res Hum Retroviruses 10: 607-616.
- Arien KK, Verhasselt B (2008) HIV Nef: Role in pathogenesis and viral fitness. Curr HIV Res 6: 200-208.
- Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, et al. (1994) Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol 68: 2906-2914.
- Aiken C, Trono D (1995) Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol 69: 5048-5056.
- Kim SY, Byrn R, Groopman J, Baltimore D (1989) Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: Evidence for differential gene expression. J Virol 63: 3708-3713.
- Klotman ME, Kim S, Buchbinder A, DeRossi A, Baltimore D, et al. (1991) Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc Natl Acad Sci USA 88: 5011-5015.
- Robert-Guroff M, Popovic M, Gartner S, Markham P, Gallo RC, et al. (1990) Structure and expression of tat-, rev- and nef-specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. J Virol 64: 3391-3398.
- Kestler HW, Ringler DJ, Mori K, Panicali DL, Sehgal PK, et al. (1991) Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65: 651-662.
- Schwartz O, Marechal V, Danos O, Heard JM (1995) Human immunodeficiency virus type 1 nef increases the efficiency of reverse transcription in the infected cell. J Virol 69: 4053-4059.
- Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB (1994) The human immunodeficiency virus-1 nef gene product: A positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med 179: 101-113.
- Sinclair E, Barbosa P, Feinberg MB (1997) The nef gene products of both simian and human immunodeficiency viruses enhance virus infectivity and are functionally interchangeable. J Virol 71: 3641-3651.
- Heigele A, Schindler M, Gnanadurai CW, Leonard JA, Collins KL, et al. (2012) Down-modulation of CD8alphabeta is a fundamental activity of primate lentiviral nef proteins. J Virol 86: 36-48.
- Laguette N, Bregnard C, Benichou S, Basmaciogullari S (2010) Human immunodeficiency virus (HIV) type-1, HIV-2 and simian immunodeficiency virus nef proteins. Mol Aspects Med 31: 418-433.
- Zou W, Lackner AA, Simon M, Durand-Gasselin I, Galanaud P, et al. (1997) Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol 71: 1227-1236.
- Stebbings RJ, Almond NM, Stott EJ, Berry N, Wade-Evans AM, et al. (2002) Mechanisms of protection induced by attenuated simian immunodeficiency virus. Virology 296: 338-353.
- Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, et al. (2012) Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 18: 1673-1681.
- Nixon DF, Donahoe SM, Kakimoto WM, Samuel RV, Metzner KJ, et al. (2000) Simian immunodeficiency virus-specific cytotoxic T lymphocytes and protection against challenge in rhesus macaques immunized with a live attenuated simian immunodeficiency virus vaccine. Virology 266: 203-210.
- National Research Council (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington DC.
- Kaizu M, Borchardt GJ, Glidden CE, Fisk DL, Loffredo JT, et al. (2007) Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics 59: 693-703.
- Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, et al. (2007) Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol 81: 8827-8832.
- Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI (1997) A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50: 657-661.
- Van Rompay KK, Singh RP, Pahar B, Sodora DL, Wingfield C, et al. (2004) CD8+-cell-mediated suppression of virulent simian immunodeficiency virus during tenofovir treatment. J Virol 78: 5324-5337.
- Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, et al. (2006) The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 80: 5074-5077.
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, et al. (2003) Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol 77: 3099-3118.
- Prete GQ, Smedley J, Macallister R, Jones GS, Li B, et al. (2015) Comparative evaluation of co-formulated injectable combination antiretroviral therapy regimens in simian immunodeficiency virus-infected rhesus macaques. AIDS Res Hum Retroviruses.
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, et al. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523-527.
- Mattapallil JJ, Letvin NL, Roederer M (2004) T-cell dynamics during acute SIV infection. AIDS 18:13-23.
- Nishimura Y, Igarashi T, Buckler-White A, Buckler C, Imamichi H, et al. (2007) Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in Simian immunodeficiency virus-infected macaques. J Virol 81: 893-902.
- Alexander L, Illyinskii PO, Lang SM, Means RE, Lifson J, et al. (2003) Determinants of increased replicative capacity of serially passaged simian immunodeficiency virus with nef deleted in rhesus monkeys. J Virol 77: 6823-6835.