Research Article - (2023) Volume 14, Issue 9
Received: 28-Sep-2022, Manuscript No. AASRFC-22-14448; Editor assigned: 30-Sep-2022, Pre QC No. AASRFC-22-14448 (PQ); Reviewed: 14-Oct-2022, QC No. AASRFC-22-14448; Revised: 03-Jan-2023, Manuscript No. AASRFC-22-14448 (R); Published: 10-Jan-2023, DOI: 10.36648/0976-8610.14.1.108
Medicinal plants play a vital role in the healthcare sector for developing nations and are potent sources of therapeutic molecules to heal various diseases in the world. Bergenia stracheyi Engl. are well known for their medicinal value in the Indian system of medicine, modern pharmaceutical industries and the local system of medicine leading to over and illegal exploitation and habitat destruction, the species is now restricted to few pockets and facing threats to their existences. Keeping this in mind, studying the different sites of B. stracheyi to get more information to take up their conservational strategies. The present study deals with variation in a number of phytoconstituents and soil organic carbon and NPK content of the plant in two different elevations. The sample result reveals that total nitrogen content and total organic carbon content increase with altitude while available nitrogen content and potassium content decrease with increasing altitude. The primary metabolites such as soluble sugar and starch increased with the elevation while protein and amino acid decreased with the elevation. The result reveals that the amount of total phenolic content and overall tannin content increase with increasing altitudes.
B. stracheyi; Ethnobotany; Photochemistry; Soil nutrient analysis; Secondary metabolites
Aqu: Aqueous; Met: Methanol; TPC: Total Phenolic Content; TFC: Total Flavonoid Content; TTC: Total Tannin Content; TAC: Total Alkaloid Content; AA: Amino Acid; PC: Phenolic Content; TPC: Total Phenolic Content; TFC: Total Flavonoid Content; TTC: Total Tannic Content; TAC: Total Alkaloid Content; AQ: Aqueous; M: Methanol
The elevation gradient is one of the environmental factors key that affect the growth, morphology, physiology and biochemistry of plants. The elevational gradient in alpine regions provides a sharp environmental change across relatively short spatial distances because small elevation changes can lead to a large shift in temperature, humidity, exposure and concentration of atmospheric gases. Thus, alpine environments can provide useful natural avenues to investigate the response of plants to a suite of climatic conditions that are representative of the broader latitudinal range. Altitude is one such factor that has been reported to bring about changes in the number of phytoconstituents. Certain metabolites are only synthesized their contents significantly increase/decrease under specific environments. Furthermore, previous reports have demonstrated that herbs grown in different environments produce variation in secondary metabolites, resulting in differences in their healing properties. There was a correlation between soil chemistry and elevation. Soil pH may also control biotic factors such as activity and biomass composition. The change in altitudinal gradients influences soil organic matter by controlling soil water balance, soil erosion, geologic deposition processes and species and biomass production of the native vegetation and cultivated plants. Soil serves as a basic growth medium for the sustained growth and development of plants in nature. Soil is one of the most important abiotic variables for the growth and development of vegetation on any land. Medicinal plants play a vital role in the healthcare sector for developing nations and are potent source of therapeutic molecules to heal various diseases in the world. Himalaya is considered the largest and most accessible source of collection of wild plants including wild fruits and medicines. Many of the plant species are restricted to small pockets and the population size of medicinally important species is decreasing at an alarming rate. The genus Bergenia belongs to the family Saxifragaceae. This family comprises 30 genera and 580 species, mostly distributed in the cold and temperate Himalayas and Central and Eastern Asia between 4000 to 12000 feet. The plant is known as Paashanbheda (Paashan=Rockstone, bheda=piercing) in hindi and rock-foil in english which itself indicates that the Bergenia plants grow between rocks and appear to break them or that they have lithotripter properties. They are clump-forming, evergreen perennials with a spirally arranged rosette of leaves 6 cm-35 cm long and 4 cm-5 cm broad and pink flowers produced in a cyme. The leaves are large, leathery, ovate or cordate and often have wavy or saw-toothed edges. For most of the year, the leaves have a glossy green color, but in cooler climates, they turn red or bronze in the fall. The Bergenia species have been used in folklore and the indigenous system of medicine for various ailments. The rhizomes and roots are cooling, bitter, acrid, laxative, astringent, abortifacient, tonic, analgesic, aphrodisiac and are useful in the treatment of tumors, heart diseases, urinary discharge, piles, spleen enlargement, ulcers, pulmonary affection, dysuria, disease of bladders, dysentery, menorrhagia, hydrophobia, biliousness, eyesores, diseases of lungs and liver and cough and fever in Unani and ayurveda system of medicine. In the folklore system of medicine, they are very effective in dissolving kidney and urinary bladder stones. The rhizomes are pulverized and made into a paste which is applied for 3-4 days on the burnt parts of the body for soothing relief. The burn heals without leaving any scar. This study aimed to determine the elevation effect on the soil parameters and quantity of biochemical and secondary metabolites of B. stracheyi [1-7].
Study Sites
Two study sites were selected from the Rudraprayag district of Uttarakhand i.e., Baniyakund (2582 m) located between latitude 30.4843° North; longitude 79.2170° East and Tungnath (3460 m) located between latitude 30.4887° N; longitude 79.2170° E according to the species natural distribution in Garhwal himalaya (Figure 1).
Figure 1: The geographical map.
Sample Collection
The studied material was collected during July 2022 from both selected sites. The upper part of the plant was collected and marked properly. For soil analysis, 6 composite soil samples were randomly collected from each site at the depth of 0 cm-20 cm and mixed thoroughly to minimize the errors. Thereafter, the samples were packed and carried out to the laboratory for future work [8-13].
Laboratory Analysis
The collected soil samples were shade dried and ground to pass through a 2 mm sieve. The pH of soil samples was determined by a pH meter. Available nitrogen in the soil was estimated by the alkaline permanganate method (Insert method). Total nitrogen was estimated by the micro Kjeldahl method as per the procedure suggested by AOAC. Measurement of the quantitative determination of phosphorous is done with the help of a spectrophotometer. Potassium contents in digested samples were determined by a flame photometer. Soil organic carbon was determined [14-16].
Preparation of Bergenia stracheyi Plant Extract
The collected plant materials were washed with tap water to remove soil, dirt and other adhering materials and cut into small pieces. The plant material was then shadows dried for one month at room temperature. The dried samples were ground in the electric grinder to get coarse powder. The dried and coarse plant materials were extracted with 100% distilled water (100°C) and 100% methanol (62°C). Methanol and water were evaporated by using a rotary evaporator, leaving a small amount of extract (2 mL-3 mL) [17,18].
Quantitative Estimation
Estimation of pigment content: The chlorophyll content in the sample was estimated and calculated using the following formulas given by Arnon.
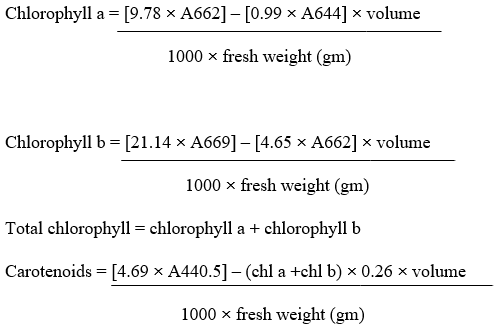
Under primary metabolite parameters soluble protein estimation was done by Bradford method, carbohydrates estimation was done by method described by Mc Cready, et al. and total amino acid estimation was done by method described Moore and Stein.
Estimation of Secondary Metabolites
Total phenolic content: The total phenolic content of dry extracts was performed with Folin-Ciocalteu’s assay 0.5 ml of sample (1 mg/ml) was mixed with 1 ml of Folin-Ciocalteu’s phenol reagent. After 5 minutes, 10 ml of 7% sodium carbonate solution was added to the mixture followed by the addition of 13 ml of deionized distilled water and mixed thoroughly. The mixture was kept in the dark for 90 minutes at 23°C, after which the absorbance was read at 760 nm. The total phenolic content was determined from extrapolation of calibration curve which was made by preparing gallic acid solution [19,20].
Total Flavonoid Content
Total flavonoid content present in both solvent extract were quantify through aluminium chloride colorimetery method followed the protocol. 50 μl of extract (1 mg of crude extract in 1 ml of solvent) was mixed with 1.5 ml of methanol. Then 0.1 ml of 10% of aluminium chloride, 0.1 ml of 1M sodium hydroxide was added. After that 2.8 ml of distilled water was added and incubated for 30 minutes in dark. The absorbance was measured at 430 nm. Quercetin were used as standard and expressed in the total flavonoid content as mg QEC/gm [21,22].
Total Tannin Content
The total tannin content was determined by adapting the Folin-Ciocalteu method. About 0.1 ml of the sample was added to a volumetric flask containing 7.5 ml of distilled water and 0.5 ml of Folin-Ciocalteu phenol reagent, 1 ml of 35% sodium carbonate solution and dilute to 10 ml with distilled water. The mixture was shaken well and kept at room temperature for 30 min. A set of reference standard solutions of tannic acid (20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml, 100 μg/ml) were prepared. Absorbance for test and standard solutions were measured against the blank at 700 nm with a UV/Visible spectrophotometer (Model no). The tannin content was expressed as milligram Tannic acid equivalents per gram of dried sample [23,24].
Total Alkaloid Content
The estimation of total alkaloid content was determined by the method given by Manjunath et al. A part of the extract residue was dissolved in 2 ml of 2N HCL and then filtered. 1 ml of this solution was transferred to a separate funnel and washed with 10 ml chloroform (3 times). The pH of this solution was adjusted to neutral with 0.1 N NaOH. Then 5 ml of bromocresol green solution and 5 ml of phosphate buffer were added to this solution. The mixture was shaken and complex extracted with 1, 2, 3 and 4 ml chloroform by vigorous shaking, the extract was then collected in a 10 ml volumetric flask and diluted with chloroform. The absorbance of the complex in chloroform was measured at the spectrum of 470 nm in UV-Spectrophotometer. Atropine was used as the standard for the calibration curve. Statistics analysis: Multiple Analysis of Variance (MANOVA) and Karl-Pearson correlation analysis were done by using IBM SPSS software (SPSS version 25) and R studio software (version 3.6.2). Data analysis was also done by using MS Excel 2007 [25-29].
Soil Parameter Analysis
The soil of both Tungnath and Baniyakund was found acidic. In Tungnath average soil pH was 5.67 whereas in Baniyakund total pH of the soil was 6.14 which were more inclined toward the neutral value of pH. Significantly maximum available nitrogen was recorded in the soil of Baniyakund (538.94 Kgha-1), followed by Tungnath (225.62 kg ha-1) however the total nitrogen content was recorded as maximum in the soil of Tungnath (2.79%) site significantly in comparison to the soil of Baniyakund (1.59%). The amount of total potassium in soil was found maximum in Baniyakund (50.94 kg ha-1) and minimum in Tungnath (44.56 kg ha-1). Similarly highest organic carbon content was found in site Tungnath (0.22 %) and the lowest in site Baniyakund (0.18%). The maximum amount of total phosphorus was recorded in the soil of Tungnath (48.72 kg ha-1) and the minimum was recorded in the soil of Baniyakund (37.54 kg ha-1) (Table 1).
| Site | Total nitrogen content | Available nitrogen content | Total organic carbon content | Total phosphorus content | Total potassium content |
|---|---|---|---|---|---|
| Tungnath | 2.79% | 225.62 kg ha-1 | 0.22% | 48.72 kg ha-1 | 44.56 kg ha-1 |
| Baniyakund | 1.59% | 538.94 Kgha-1 | 0.18% | 37.54 kg ha-1 | 50.94 kg ha-1 |
Table 1: Comparison of soil nutrients from Tungnath and Baniyakund.
Biochemical Analysis
Environmental factors strongly affect the metabolism and accumulation of phytoconstituents. The quality and quantity of active constituents are regulated by most plants depending on the environmental conditions. In this study, significant differences were observed in the quantities of biochemical and active phytoconstituents in Bergenia stracheyi. Total chlorophyll content was recorded highest at Tungnath with increasing quantities of chlorophyll a (1.22 mg/gm), chlorophyll b (1.66 mg/gm) and carotenoid (2.78 mg/gm) whereas, at Baniyakund, decreasing quantities of chlorophyll a (1.12 mg/gm), chlorophyll b (1.54 mg/gm) and carotenoids (2.00 mg/gm) were recorded. Total chlorophyll content was recorded highest at Tungnath (2.88 mg/gm) and the minimum was recorded at Baniyakund (2.66 mg/gm). Estimation of carbohydrates was done in two parts: first for soluble protein and second for total starch content. Soluble sugar was recorded highest at Tungnath (322.66 mg/gm) whereas at Baniyakund it was recorded low (297.33 mg/gm). The starch content with a maximum at Tungnath (183 mg/gm) and the minimum value was recorded at Baniyakund (43.3 mg/gm). The variation in the protein content of the leaf was negatively correlated with the altitude. The maximum value was recorded at Baniyakund (16.29 mg/gm) and the minimum value was recorded at Tungnath (12.83 mg/gm). Total free amino acid decreased with the increasing elevation. The highest value was recorded at Baniyakund (108.33 mg/gm) and the minimum at site Tungnath (101.66 mg/gm) (Table 2).
| Population | Total chloroph yll content | Total carotenoid content | Total soluble protein content | Total soluble sugar content | Total starch content | Total free amino acid content | Total phenolic content (fresh sample) |
|---|---|---|---|---|---|---|---|
| Baniyakund | 2.66 mg/gm | 2.78 mg/gm | 16.29 mg/gm | 297.33 mg/gm | 43.3 mg/gm | 186.33 mg/gm | 72.02 mg/gm |
| Tungnath | 2.88 mg/gm | 2.00 mg/gm | 12.83 mg/gm | 322.66 mg/gm | 183 mg/gm | 104.33 mg/gm | 90 mg/gm |
Table 2: Quantitative analysis of biochemical constituents of Bergenia stracheyi.
Karl-Pearson correlation coefficients among different biochemical variables and altitude indicated that carotenoid content (r=-0.936, p<0.01) and soluble sugar ((r=-0.929, p<0.01) content were negatively correlated with altitude, however protein (r=0.936, p<0.01) and starch (r=0.823, p<0.05) were positively correlated with the altitude.
Secondary Metabolites Analysis
In this study, significant differences were observed in the number of active constituents in the whole plant of B. stracheyi growing in the region of two different altitudes. The amount of Total phenolic content, total lavonoid content and total tannin content present in the plant collected from higher altitudes were more whereas total alkaloid content was higher in the plant collected from the lower altitude (Table 3).
| Sites | TPC mgGAE/g | TFC mgQEC/g | TTC mgTAC/g | TAC mgATC/g | ||||
|---|---|---|---|---|---|---|---|---|
| Aqu | Met | Aqu | Met | Aqu | Met | Aqu | Met | |
| Tungnath | 90.7 ± 1.3** | 85.5 ± 2.1* | 6.25 ± 0.2* | 4.18 ± 0.3* | 9.07 ± 0.4** | 28.05 ± 0.5* | 66.1 ± 5.6* | 60.1 ± 6.3** |
| Baniyakund | 70.0 ± 1.4* | 97.6 ± 1.5* | 5.11 ± 0.3* | 13.5 ± 0.3* | 4.80 ± 0.02* | 29.66 ± 6.4* | 44.22 ± 3.7* | |
| Note: *= significant at 0.05, **=significantly at 0.01 | 29.66 ± 6.4* | |||||||
Table 3: Quantitative analysis of secondary metabolite in aqueous and methanolic extract of B. stracheyi at both elevations.
Karl-Pearson correlation coefficients among secondary metabolites and altitude indicated that aqueous extract of total phenolic content ((r=-0.994, p<0.01), flavonoid content (r=-0.925, p<0.05), total tannin content (r=-0.993, p<0.01) and methanolic extract of total alkaloid content (r=-0.946, p<0.01) were negatively correlated with altitude however methanolic extract of total phenolic content (r=0.970, p<0.01) and aqueous extract of total alkaloid content (r=0.941, p<0.01) were positively correlated with altitude (Table 4).
| Altitude | chl a | chl b | Carotenoid | Protein | Soluble sugar | Starch | AA | PC | TPC (AQ) | TPC (M) | TFC (AQ) | TFC (M) | TTC (AQ) | TTC (M) | TAC (AQ) | TAC (M) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude | 1 | ||||||||||||||||
| chl a | 0.426 | 1 | |||||||||||||||
| chl b | 0.295 | .923** | 1 | ||||||||||||||
| Carotenoid | 0.81 | 0.461 | 0.215 | 1 | |||||||||||||
| Protein | -0.768 | -0.285 | -0.102 | -0.634 | 1 | ||||||||||||
| Soluble sugar | 0.568 | 0.196 | 0.219 | 0.572 | 0.043 | 1 | |||||||||||
| Starch | .997** | 0.46 | 0.311 | .835* | -0.801 | 0.528 | 1 | ||||||||||
| AA | -.996** | -0.397 | -0.247 | -0.803 | 0.766 | -0.551 | -.992** | 1 | |||||||||
| PC | .850* | 0.457 | 0.276 | .817* | -0.399 | 0.769 | .837* | -.864* | 1 | ||||||||
| TPC (AQ) | -.994** | -0.399 | -0.233 | -.820* | 0.8 | -0.513 | -.994** | .998** | -.849* | 1 | |||||||
| TPC (M) | .970** | 0.391 | 0.276 | 0.744 | -.881* | 0.392 | .976** | -.961** | 0.701 | -.968** | 1 | ||||||
| TFC (AQ) | -.925** | -0.509 | -0.42 | -0.583 | 0.776 | -0.334 | -.922** | .926** | -0.709 | .921** | -.936** | 1 | |||||
| TFC (M) | -0.769 | -0.419 | -0.343 | -0.753 | .829* | -0.291 | -0.799 | 0.726 | -0.436 | 0.748 | -.848* | 0.661 | 1 | ||||
| TTC (AQ) | -.993** | -0.458 | -0.34 | -.830* | 0.696 | -0.656 | -987** | .985** | -.886* | .978** | -941** | .895* | 0.754 | 1 | |||
| TTC (M) | -0.024 | 0.406 | 0.275 | 0.522 | 0.103 | 0.296 | 0.018 | 0.058 | 0.192 | 0.038 | -0.102 | 0.274 | -0.26 | -0.048 | 1 | ||
| TAC (AQ) | .941** | 0.502 | 0.381 | 0.647 | -0.677 | 0.476 | 932** | -.952** | 0.843 | -.941** | .897* | .972** | -0.568 | .928** | -0.207 | 1 | |
| TAC (M) | -.946** | -0.574 | -0.394 | -0.768 | .859* | -0.32 | -.962** | .947** | -0.761 | .957** | -.953** | .954** | 0.76 | .917** | 0.032 | -.938** | 1 |
| Note: *=significant at 0.05 level. **=significant at 0.01 level. | |||||||||||||||||
Table 4: Correlation between different primary metabolites, secondary metabolites and altitudes of B. stracheyi.
Soil has a close relationship with geomorphology and the vegetation type of the area. However, variability in soil characteristics even within the same geomorphic position has also been reported. At the alpine sites of B. stracheyi, the soil was acidic. In general soil at high altitudes is known to be acidic. Soil mineralogical information is important for soil fertility evaluation. The major source of soil nitrogen is organic materials. Alpine plants are often adapted to low nitrogen content and it has long been admitted that cold regions are inadequate in nutrients. Available nitrogen was recorded as a maximum at Baniyakund and minimum at Tungnath. Soil N mineralization and nitrification rates decreased with altitude and this is because the altitude affects mountain vegetation by directly influencing the solar radiation and soil moisture. Due to decreasing temperature, respiration rates decrease at higher altitudes which lead to the enhancement of carbon and nitrogen in the soil. Similar trends were recorded for phosphorus content in all the populations of both species. Phosphorus content was observed at maximum at Tungnath and minimum at Baniyakund. The low amount of phosphorus in soil reflects the characteristics of the soil to permit the plant to grow in a particular area and determine the type of vegetation of the area. Among all the organisms living on the earth, plants are much more diverse as they adapt to their environment much more readily. Plants can’t move so they have to develop strategies for adaptation to their surrounding environment for survival. Plants are well known for their secondary metabolites, which are produced as by-products in almost all important primary biochemical pathways. The present study was planned to keep in mind that the changing altitude may affect the number of different biochemical and phytochemicals and their activity of B. stracheyi. The plants thriving under different environmental conditions face stress of various types and degrees, so to relieve unpleasant conditions, it develops their defense mechanism by accumulating various secondary metabolites. With increasing altitude, the total chlorophyll content increased. Both the chlorophyll a and chlorophyll b concentrations were increased with the altitudes in both species. Carotenoid content seemed to increase with altitude. This may be due to the reasons reported that light is necessary for carotenoid synthesis. Hence, the enhancement in carotenoid contents increasing altitude may be attributed to the protective function of the pigment in the dissipation of excess energy and scavenging of free radicals. In B. stracheyi soluble sugar increase with the increase of elevation. An increasing trend in the soluble sugar is observed along the altitude as populations at high altitudes need to cope with persistent freezing temperatures so that they accumulate higher soluble sugar providing resistance to this low temperature. In B. stracheyi amino acid increase with the increase of elevation. The increase in total amino acid content along the altitudinal gradient is a common phenomenon of plant species at high altitudes, as these play an important role in dealing with various stresses as the accumulation of free amino acid and proline plays an important role in osmoregulation. In B. stracheyi dependent variable like a leaf, the protein was not found significantly correlated with the altitude. It means that some other factors other than altitude are responsible for these variations like the climatic variables that bring the changes at the microhabitat level of the species by altering the available soil nutrients level. The increasing demand for therapeutically important secondary metabolites can be securely met if we can map the variation in their content in plants owing to changes in seasons and altitudes. Thus the evaluation of altitudinal variation in plant secondary metabolites, triggered by ecologic factors varying with the altitude of the growing site, is very important. The present study reveals that the total phenolic content, total flavonoid content and total tannin content increased with altitude. The highest TPC and TFC were observed in the methanolic extract of B. stracheyi at Tungnath as compared to the methanolic extract of Baniyakund. This increase in phenolic content with an increase in altitude may be attributed to a response to plants to enhance UV B radiation and decreased temperatures which elicit amplified biosynthesis of UV absorbing and antioxidant phenolic in plants. Total tannin content increase with the increasing elevation which may be due to the adaptation to frost-resistant cells, to avoid any injury during unfavorable temperate conditions documented increased tannin compound in water stress as a consequence of low temperature. Total alkaloid content was decreased with the increasing elevation. There exists compelling evidence about the increasing pressures caused by herbivores at lower elevations; hence the increase in alkaloid content may be regarded as an obvious defense measure adopted by the plants at lower elevations [25].
The present study suggests that screening of multilocational plants/populations is essential for optimizing suitable sources for higher phytochemicals. The species B. stracheyi was taken into consideration across the specified location (Baniyakund and Tungnath) of Garhwal himalaya and showed considerable variation in the soil parameters and biochemical characteristics (primary and secondary metabolites). This study’s results revealed that the changes in altitude had a significant impact on certain physico-chemical properties of soil. The future trend of the study plays an important role in understanding the change in soil property effects on plant photochemistry and the genetic diversity of plants in the area. The plants may be considered a biosynthetic laboratory for a variety of compounds (secondary metabolites) like alkaloids, glycosides, flavonoids, volatile oils and saponins that exert physiological effects. The curative properties of medicinal plants are due to the presence of various secondary metabolites. A phytochemical study was also useful to isolate the pharmacologically active principles, present in the drug. More phytochemical research work is required for the isolation, purification and characterization of biological compounds. The selected medicinal plants are the source of the secondary metabolites i.e., alkaloids, flavonoids, tannins and phenolic. Medicinal plants play a vital role in preventing various diseases. The antidiuretic, anti-inflammatory, antianalgesic, anticancer, anti-viral, anti-malarial, anti-bacterial and anti-fungal activities of the medicinal plants are due to the presence of the above-mentioned secondary metabolites. Medicinal plants are used for discovering and screening the phytochemical constituents which are very helpful for the manufacturing of new drugs. The phytochemical analysis of medicinal plants is also important and has a commercial interest in both research institutes and pharmaceutical companies for the manufacturing of new drugs for the treatment of various diseases. The species has the potential to enhance the income of the Himalayan people; therefore, a breeding program for this species needs to be initiated and material for the same can be used from the identified promising sources.
The authors are very thankful to acknowledge the director HAPPRC, HNB Garhwal University Srinagar for providing the facility and guidance for this study.
Writers do not have any conflict of interest to declare.
JT was involved in the study design, data collection, manuscript preparation and manuscript writing and investigation vs. contributed to the statistical analysis, writing a review, editing and reviewing.
Citation: Thapliyal J, Shukla V (2023) The Elevational Effect on Soil Parameters and Biochemical Constituents of Bergenia stracheyi: An Important Medicinal Plant of Garhwal Himalaya. Adv Appl Sci Res. 14.108
Copyright: �???????�??????�?????�????�???�??�?�© 2023 Thapliyal J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.