- (2011) Volume 12, Issue 6
Ho Kyoung Hwang1, Joon Seong Park1, Chan-il Park2, Jae Keun Kim1, Dong Sup Yoon1
Departments of 1Surgery and 2Pathology, Yonsei University College of Medicine. Seoul, South Korea
Received July 28th, 2011 - Accepted September 16th, 2011
Context Several surgical complications are related to obesity. Objective This study evaluated the impact of obesity on pancreatic fistula after pancreaticoduodenectomy. Design We retrospectively reviewed the medical records of 159 patients who underwent pancreaticoduodenectomy between October 2002 and December 2008. Setting The patients were divided according to the body mass index as obese (body mass index equal to, or greater than, 25 kg/m2), or normal (body mass index less than 25 kg/m2). Methods Univariate and multivariate analyses were applied. Two-tailed P values less than 0.05 were considered as significant. Results Forty-six patients (28.9%) were obese and 113 patients (71.1%) were normal-weight. Obese group had a significantly higher incidence of pancreatic fistula and a greater amount of intraoperative blood loss. Other surgical complications were not significantly different between the two groups. Multivariate analysis found obesity, small pancreatic duct size (less than, or equal to, 3 mm), intraoperative blood loss, and combined resection as significant factors affecting pancreatic fistula. Conclusions Obese patients have an increased risk for pancreatic fistula after pancreaticoduodenectomy.
Body Mass Index; Obesity; Pancreatic Fistula; Pancreaticoduodenectomy
ISGPF International Study Group of Pancreatic Fistula
Pancreaticoduodenectomy performed in high volume centers has become increasingly safe and more efficient. However, the morbidity rate remains high with complication rates of around 40%. The major complication leading to serious results is pancreatic fistula and well-known main risk factors of pancreatic fistula are small pancreatic duct size, and soft texture of pancreatic parenchyma [1, 2].
Obesity is rapidly becoming a major public health problem in many countries around the world. Obesity is related with several chronic diseases including diabetes mellitus, cardiovascular disease, stroke, hypertension and certain cancers. It is also associated with perioperative complications and has been considered a risk factor for surgical outcomes of patients undergoing abdominal surgery [3, 4, 5].
Soft pancreas, which is more frequently observed in obese patients, could explain why body mass index (BMI) appears as a risk factor of pancreatic fistula after distal pancreatectomy [6]. There were some studies evaluating a correlation between obesity and pancreatic fistula after pancreaticoduodenectomy in Western countries, and obesity in those studies was defined as over 30 kg/m2 of BMI [7, 8]. However, there was a debate about obesity criteria for Asian populations, and the International Association for the Study of Obesity (IASO) and the International Obesity Task Force (IOTF) proposed a BMI cut-off point of 25 kg/m2 for obesity in Asian populations [9].
The aim of this study was to evaluate the impact of obesity on pancreatic fistula after pancreaticoduodenectomy in Asian patients on the basis of Asia- Pacific perspective of BMI in obesity.
Patients
Between October 2002 and December 2008, 159 patients with benign and malignant periampullary lesions underwent pancreaticoduodenectomy by one surgeon (D.S.Y.) at the Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine.
Surgical Procedures
Pylorus preserving pancreaticoduodenectomy and conventional pancreaticoduodenectomy were performed in previously reported manners [10].
As a first step in reconstruction, the proximal jejunum was brought through the transverse mesocolon by the retrocolic route. Pancreaticojejunostomy was performed with duct-to-mucosa anastomosis in all patients. The inner layer was duct-to-mucosa with interrupted 5-0 polypropylene sutures, and a short internal stent was used. The outer layer was a seromuscular envelope with interrupted 4-0 polyglactin Lambert sutures. End-to-side hepaticojejunostomy (or choledochojejunostomy) was performed 15 cm proximal to the pancreaticojejunostomy with singlelayer interrupted sutures. An antecolic duodenojejunostomy (or gastrojejunostomy) was constructed using a two-layered anastomosis. After reconstruction, a tube gastrostomy was routinely performed instead of using a nasogastric tube. Tube gastrostomy was performed as follows: a small gastrostomy was made in the anterior wall of the stomach, and an 18 Fr balloon catheter was inserted. The tube was exteriorized through the abdominal wall and was fixed to the skin. A closed suction, silicon drain (Jackson-Pratt, Baxter Health Care Corp., Deerfield, IL, USA), was placed from the right upper quadrant posterior to the pancreaticojejunal and biliary anastomosis.
Assessment of Pancreas Consistency
In all cases, pancreatic duct diameter was measured after pancreatic transection by the operator. Parenchyma texture was divided into two groups based upon the extent of fibrotic changes in the resection margin. A fibrotic change was assessed by trichrome staining. Soft pancreatic parenchyma was characterized by the absence of fibrosis or slight thickness of perilobular fibrosis (up to 50 μm). Hard parenchyma was characterized by thick perilobular fibrosis greater than 50 μm (Figure 1).
Figure 1. Results of trichrome staining on the resection margin of the pancreas. a. b. Absence of fibrosis or slight thickness of perilobular fibrosis (less than, or equal to,50 μm ). c. d. Hard parenchyma was characterized by thick perilobular fibrosis greater than 50 μm. The purple color stained by trichrome show the component of fibrotic change in perilobular space.
Perioperative Management
According to the department policy, all patients received anti-acid drugs for stress ulcer prophylaxis, and octreotide (Sandostatin® 0.1 mg; Novartis International, Basel, Switzerland) was administered subcutaneously for 7 days postoperatively. Gastric suction through the gastrostomy tube was stopped within the first 3 postoperative days. After postoperative day 3, the gastrostomy tube was clamped for 24 hours, and patients were given sips of water between postoperative days 4 and 5. Patients then proceeded to a regular diet within 7 days.
Classification of Pancreatic Fistula and Delayed Gastric Emptying
Pancreatic fistula is defined by output via an operative drain of any measurable volume of drain fluid on or after postoperative day 3 with amylase content greater than three times the upper normal serum level (more than 300 IU/L) according to the definition given by the International Study Group of Pancreatic Fistula (ISGPF) [11]. The grade of pancreatic fistula was divided as grade A, B and C according to the severity of pancreatic fistula. And we redefined the pancreatic fistula according to the clinical importance. Clinically relevant pancreatic fistula including grade B and C were redefined as ‘Clinical Yes’ group, the other group including absence of pancreatic fistula and grade A were redefined as ‘Clinical No’ group.
The severity of delayed gastric emptying was determined according to the classification scheme proposed by the International Study Group of Pancreatic Surgery (ISGPS) [12]. In this classification, delayed gastric emptying is defined by the need for maintenance of nasogastric tube for 3 days, need for reinsertion of nasogastric tube for persistent vomiting after postoperative day 3, or inability to tolerate a solid diet by postoperative day 7. The present study evaluated delayed gastric emptying based on tube gastrostomy period instead of nasogastric tube period.
This study was approved by the Institutional Review Board of Yonsei University for retrospective chart review and data collection. Any written or oral informed consent was not obtained from each patient. However, the study protocol conformed to the ethical guidelines of the “World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects”.
Continuous variables are expressed as the mean ± standard deviation (SD) or median and interquartile range (IQR) with skewed distributions. Differences between groups were analyzed by using the Mann- Whitney, the linear-by-linear association chi-square, or the Fisher’s exact tests. To test the independence of the risk factors, all variables tested in univariate analysis but hospital stay (because it was not an influencing factor, but just a result of pancreatic fistula) were entered into a stepwise multivariate nonconditional logistic regression model and the odds ratios and the 95% confidence intervals were also evaluated. Twotailed P values less than 0.05 were considered significant.
A total of 159 patients who underwent pancreaticoduodenectomy (or pylorus preserving pancreaticoduodenectomy) were selected for this study. The average age (±SD) of patients was 60.2±11.9 years, and 90 of them (56.6%) were men. The median BMI was 23.4 kg/m2 (IQR: 21.5-25.3 kg/m2), the median intraoperative blood loss was 1,100 mL (IQR: 700- 1,600 mL), the median operative time was 425 min (IQR: 375-530 min), and the median duration of hospital stay was 24 days (IQR: 21-31 days).
Fifty-one patients (32.1%) underwent pancreaticoduodenectomy, and 108 (67.9%) underwent pylorus preserving pancreaticoduodenectomy. Pathologic diagnoses were pancreatic carcinoma in 45 patients (28.3%), benign pancreas disease (chronic pancreatitis or pancreatic cystic neoplasms) in 15 (9.4%), bile duct cancer in 45 (28.3%), benign biliary disease in 3 (1.9%), ampulla of Vater carcinoma in 35 (22.0%), adenoma of the ampulla of Vater in 7 (4.4%), duodenal carcinoma in 8 (5.0%), and pancreas head trauma in 1 (0.6%).
Forty-six patients (28.9%) were obese which is defined as BMI over 25 kg/m2, and 113 patients (71.1%) were normal-weight as defined by the Asia-Pacific perspectives criteria [6].
Demographic and clinical data of both groups are shown in Table 1. The two groups showed no significant differences in age, sex, comorbidity, size of pancreatic duct and texture of pancreas parenchyma. However, there was significantly larger amount of intraoperative blood loss in obese group while combined resection was made in normal group only. There were no cases of hospital mortality (within 30 days).
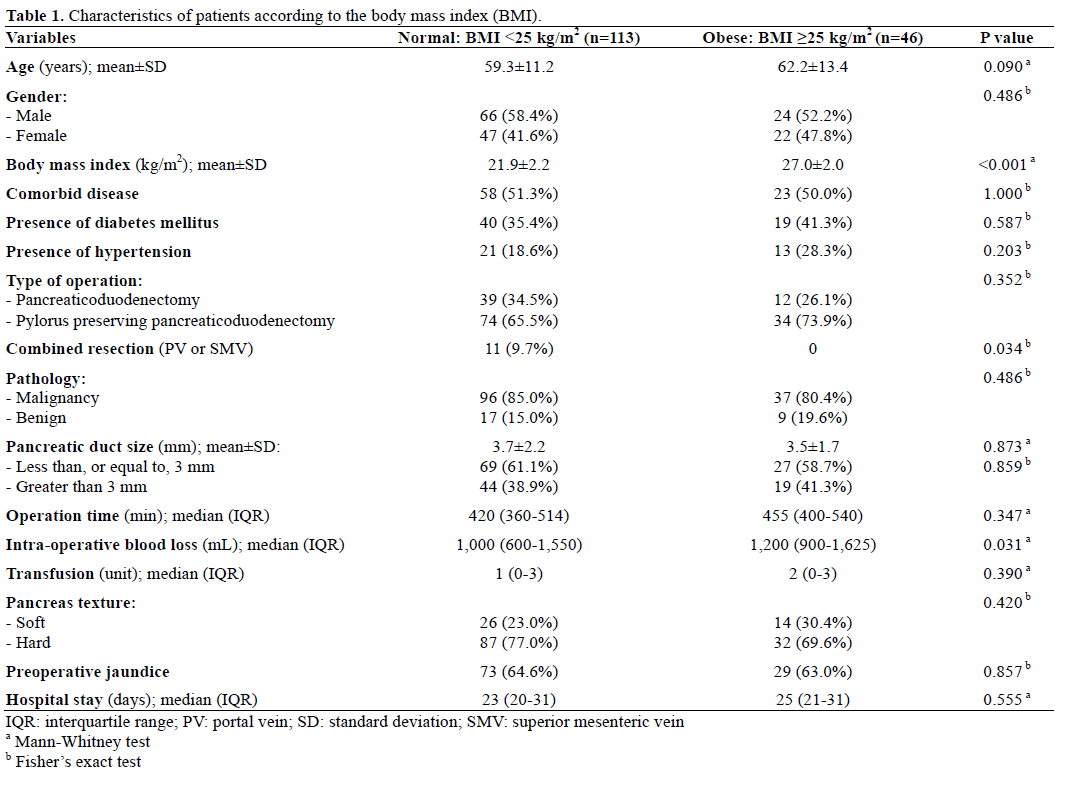
Ninety-two patients (57.9%) had one or more postoperative complications: 55 patients (34.6%) had one complication, 33 patients (20.8%) had two complications and 4 patients (2.5%) had three complications. In addition, routine abdominal CT scan at the 7th postoperative day showed simple intraabdominal fluid collection or ascites in two patients (1.3%) with BMI less than 25 kg/m2. We did not include these data as complications; one of these patients had other postoperative complications.
There was no significant difference in postoperative complications in both groups except pancreatic fistula (Table 2). Pancreatic fistula was the most common complication in this study. Sixty-three patients (39.6%) developed pancreatic fistula: 36 (22.6 %) patients had grade A, 23 (14.5 %) grade B, and 4 (2.5%) grade C. The incidence of pancreatic fistula was significantly higher in obese group (P=0.020) as well as the grade of pancreatic fistula was positively related to obesity (P=0.002).
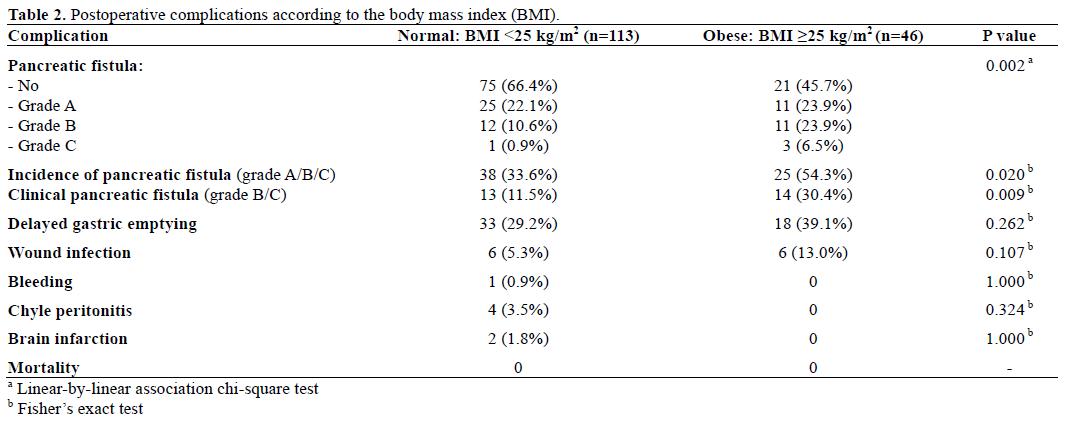
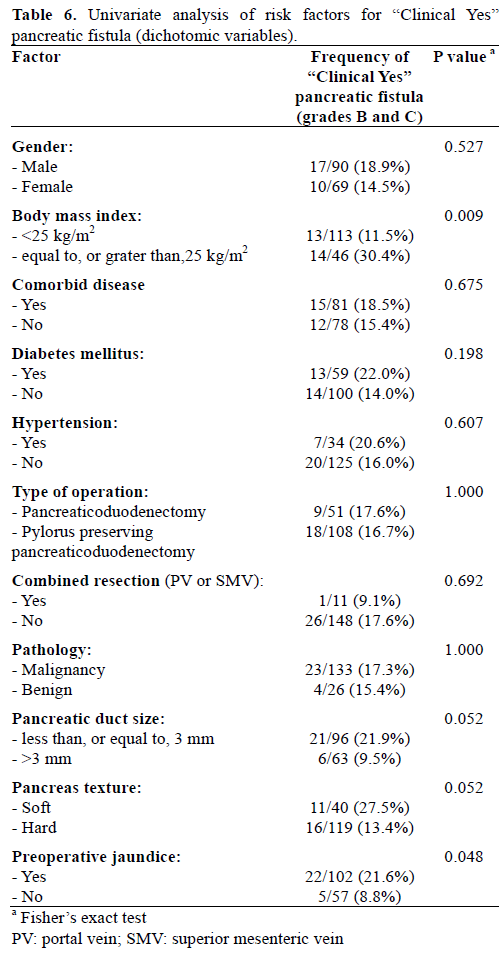
In univariate analysis, the incidence of pancreatic fistula was significantly greater in patients with BMI over 25 kg/m2, small pancreatic duct size (less, or equal to, 3 mm), and soft pancreatic texture. Patients with pancreatic fistula had also longer operative time and large amount of both intraoperative blood loss and transfusion. The rates of pancreatic fistula did not correlated with age, sex, operation method (pancreaticoduodenectomy versus pylorus preserving pancreaticoduodenectomy), preoperative medical disease history (diabetes mellitus, hypertension), pathology (benign or malignant), and preoperative jaundice (Tables 3 and 4).
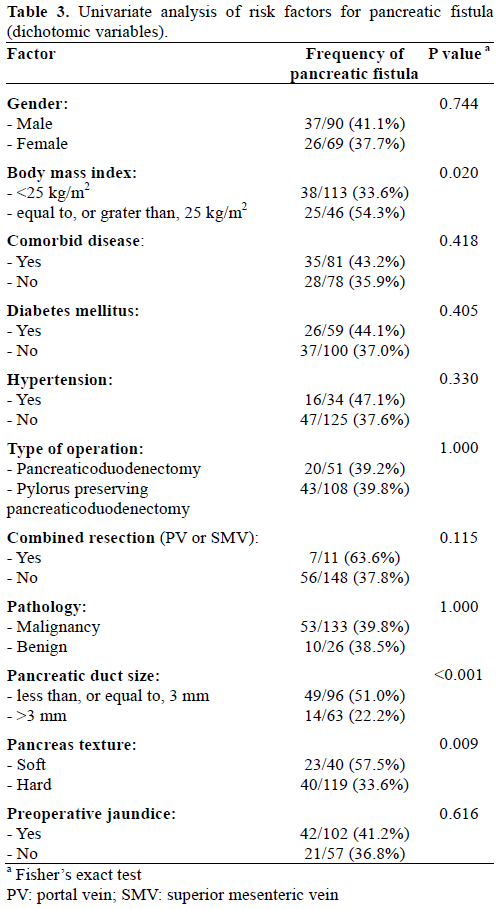

In multivariate analysis, BMI over 25 kg/m2, small pancreatic duct size (less, or equal to, 3 mm), intraoperative blood loss, and combined resection were identified as independent factors for pancreatic fistula (Table 5).
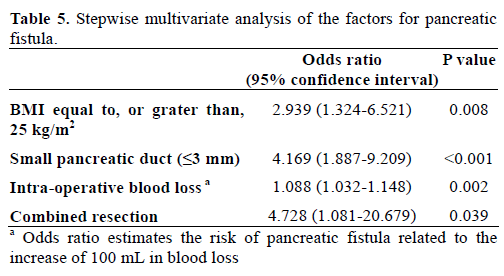
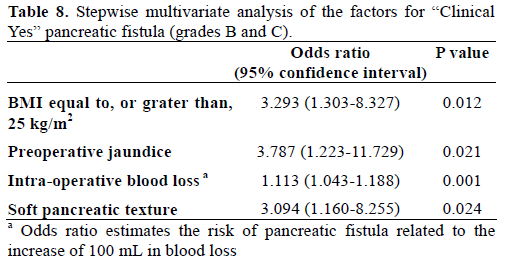
When we re-evaluated the pancreatic fistula as clinically relevant pancreatic fistula, like as ‘Clinical Yes’ group and ‘Clinical No’ group, univariate analysis showed BMI over 25 kg/m2, preoperative jaundice, longer operative time, and large amount of both intraoperative blood loss and transfusion were significantly related to clinically relevant pancreatic fistula. However, small pancreatic duct size (less, or equal to, 3 mm) (P=0.052) and soft pancreatic texture (P=0.052) had marginal significance on clinically relevant pancreatic fistula (Tables 6 and 7). In the multivariate analysis of clinically relevant pancreatic fistula, BMI over 25 kg/m2, soft pancreatic texture, and intraoperative blood loss were confirmed as independent risk factors together with preoperative jaundice, whereas small pancreatic duct did not enter this analysis (Table 8).

Public health issues related with obesity are rapidly increasing throughout Asia as well as in developed countries [13]. The World Health Organization (WHO) defines obesity based on BMI over 30 kg/m2. However, the WHO classification cannot be used for Asians because their heights and weights are different from Western population. Therefore, Asia-Pacific guidelines for obesity concluded that BMI greater than 25 kg/m2 may be considered as obesity in Asians [9]. So, we defined obesity as BMI higher than 25 kg/m2 in this study.
Actually, the prevalence rate of BMI equal to, or grater than,25 kg/m2 and BMI equal to, or grater than, 30 kg/m2 in Korean was 29.5% and 3.0 % in 2001 according to the National Health Examination and Nutrition Survey (KNHENES) data of 1998 [14] and 2001 [15, 16]. The obesity rate in our study was 28.9 % (BMI equal to, or grater than, 25 kg/m2) and 1.9% (BMI equal to, or grater than, 30 kg/m2), which was similar with the national survey.
Many reports showed that intraoperative blood loss, wound infection rate, and mortality were higher in obese patients after abdominal surgery including pancreaticoduodenectomy [3, 5, 17, 18, 19]. Our results showed that the amount of intraoperative blood loss and incidence of pancreatic fistula were significantly higher in obese patients, but there was no significant difference in other postoperative complications.
In this study, the ISGPF definition of pancreatic fistula was used [11]. Pancreatic fistula is a major factor remaining morbidity rate unchanged after pancreaticoduodenectomy, even though its mortality rate is decreased to less than 5% in high volume centers. It is difficult to evaluate the actual incidence of pancreatic fistula, because it has been defined in several ways in the literature. In some studies, grade A pancreatic fistula was not considered as pancreatic fistula. Noun R et al. [20] showed that the rate of clinically relevant pancreatic fistula (36.8% vs. 15.1%; P=0.050) was significantly increased in obese vs. non-obese patients. In that study, pancreatic fistula was defined as ISGPF grade B and C fistulas. In our study, ISGPF grade A pancreatic fistula was also considered as pancreatic fistula. Pancreatic fistula rate was significantly different between obese and normal weight patients (54.3% vs. 33.6%; P=0.020) (Table 2). If only grades B and C were redefined as pancreatic fistula, the rate would be more significantly different (30.4% vs. 11.5%; P=0.009) and this pancreatic fistula rate was similar to that observed in other studies.
Generally accepted risk factors affecting pancreatic fistula are soft pancreatic texture and small pancreatic duct without dilatation [2]. In this study, BMI equal to, or greater than, 25 kg/m2, small pancreatic duct size (less than, or equal to, 3 mm), intraoperative blood loss, and combined resection were identified as independent factors for pancreatic fistula in multivariate analysis (Table 5). When we redefined the pancreatic fistula as ‘Clinical Yes’ group including the ISGPF definition of grades B and C and ‘Clinical No’ group including ISGPF grade A and patients who did not have pancreatic fistula, in multivariate analysis, BMI equal to, or greater than, 25 kg/m2, soft pancreatic texture, intraoperative blood loss, and preoperative jaundice were significantly meaningful factors (Table 8).
Sledzianowski JF et al. reported that soft pancreas, which is more frequently observed in obese patients, could explain why BMI appears as a risk factor of pancreatic fistula after distal pancreatectomy [6]. In our results, soft pancreas was slightly more frequently observed in obese patients (30.4% vs. 23.0%; P=0.420), but there was no significant difference.
Noun R et al. [20] demonstrated that the amount of intrapancreatic fat was increased in 50% of obese patients and that this increase positively correlated with BMI. The occurrence of pancreatic fistula had a correlation with intrapancreatic fat amount (fat amount was graded from absent to massive on a four-point scale). Mathur et al. [8] proposed that patients who develop a postoperative pancreatic fistula have higher pancreatic fat levels. Lee SE et al. [21] demonstrated that intralobular, interlobular, and total fat were significantly elevated in soft pancreatic group using magnetic resonance imaging analysis and more patients in the pancreatic fistula group had a soft pancreatic texture. In our results, we did not evaluate the amount of intrapancreatic fat from surgical pathology specimens, but the textures of pancreas parenchyma, graded by thickness of perilobular fibrosis on the trichrome staining, were not different between the two groups. Also, the pancreatic duct size was not different between normal-weight and obese patients. We could not demonstrate the reason why pancreatic fistula was higher in obese patients in the respect of generally accepted risk factors (soft pancreatic texture and small pancreatic duct) in this study. However, the amount of intrapancreatic fat was proved to be positively correlated with BMI influencing soft pancreatic texture in other studies [8, 20, 21]. Another of the possible theory was that larger blood loss in obese patients impair several surgical procedures, induce longer operation time and it might negatively affect to pancreaticojejunostomy. In this study, blood loss was larger in obese patients, but the operation time was not different (Table 1).
In summary, based on retrospective evaluation of impact of obesity for pancreatic fistula after pancreaticoduodenectomy, obese patients (BMI equal to, or grater than, 25 kg/m2) have increased risk for pancreatic fistula after pancreaticoduodenectomy. Small pancreatic duct size (less than, or equal to, 3mm), intraoperative blood loss and combined resection were also identified as independent factors for pancreatic fistula. Therefore, precise operative technique to reduce intraoperative blood loss is recommended especially in obese patients who have soft pancreatic texture. However, we could not demonstrate the reason why BMI over 25 kg/m2 had the positive correlation with pancreatic fistula. More experience and clinical evaluation are necessary.
Part of this article was presented at the 65th Annual Meeting of the Japanese Society of Gastroenterological Surgery held in Shimonoseki, Japan on July 15, 2010
Ho Kyoung Hwang, Joon Seong Park, Chan-il Park, Jae Keun Kim, and Dong Sup Yoon have no conflicts of interest or financial ties to disclose