Takeo Uba1, Kenichiro Nishi1, Takeshi Umegaki1*, Naotsugu Ohashi2, Yusuke Kusaka3, Osamu Umegaki4and Shinichi Nishi2
1Department of Anesthesiology, Kansai Medical University Hospital, Osaka, Japan
2Division of Intensive Care Medicine, Hyogo College of Medicine College Hospital, Hyogo, Japan
3Department of Anesthesiology, Osaka Medical College Hospital, Osaka, Japan
4Department of Anesthesiology, Intensive Care Unit, Osaka Medical College Hospital, Osaka, Japan
*Corresponding Author:
Takeshi Umegaki
MD PhD, Department of Anesthesiology
Kansai Medical University Hospital
Osaka, Japan.
Tel: 404-783-2008; Ext: 56372
Fax: +81728042785
E-mail: umegakit@hirakata.kmu.ac.jp, ane12429take@gmail.com
Received date: October 01, 2017; Accepted date: October 11, 2017; Published date: October 18, 2017
Citation: Uba T, Nishi K, Umegaki T, Ohashi N, Kusaka Y, et al. (2017) The Influence of Human Soluble Recombinant Thrombomodulin on In-Hospital Mortality in Patients with Acute Respiratory Distress Syndrome and Disseminated Intravascular Coagulation: A Retrospective Multicenter Study. J Intensive & Crit Care Vol.3 No.4:40
Keywords
Human soluble recombinant thrombomodulin; Acute respiratory distress syndrome; Disseminated intravascular coagulation; In-hospital mortality
Abbreviations
APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: Acute Respiratory Distress Syndrome; DIC: Disseminated Intravascular Coagulation; eGFR: Estimated Glomerular Filtration Rate; HMGB-1: High-Morbidity Group Box-1; HR: Hazard Ratio; ICU: Intensive Care Unit; P/F ratio: PaO2/Fraction Oxygenation Ratio; rTM: Human Soluble Recombinant Thrombomodulin; SOFA: Sequential Organ Failure Assessment; AT: Antithrombin
Background
Acute respiratory distress syndrome (ARDS) refers to the development of acute noncardiogenic pulmonary edema due to an increase in pulmonary vascular permeability. It is not uncommon for patients with ARDS to develop disseminated intravascular coagulation (DIC) [1] and patients with DIC also develop ARDS with fairly high frequency [1,2]. The development of DIC in ARDS patients is associated with poorer prognoses, which underlines the need for early treatment [2]. Anticoagulant therapy, including antithrombin therapy and thrombomodulin therapy, has shown to be efficacious in the treatment of acute lung injury in animal experiments [3]. This suggests that anticoagulant therapy may also be effective in the treatment of human patients with ARDS in the clinical setting [3].
Thrombomodulin is a membrane protein that serves as a thrombin receptor on endothelial cells and plays an important role in regulating intravascular coagulation through the thrombomodulin-protein C pathway [4,5]. Human soluble recombinant thrombomodulin (rTM) is composed of the active extracellular domain of thrombomodulin, which forms a reversible complex with thrombin to convert plasma protein C into its activated form. This in turn inactivates coagulation factors and reduces the pro-inflammatory effects of thrombin. In addition, rTM directly binds high-mobility group box 1 (HMGB-1), thereby preventing its interaction with receptors for advanced glycation end-products and suppressing the induction of pro-inflammatory events [6]. A phase III clinical trial of rTM treatment for DIC associated with hematologic malignancies and infection reported that patients treated with rTM had a significantly higher DIC resolution rate when compared with patients treated with unfractionated heparin; however, there was no significant difference in 28-day mortality between the 2 treatment modalities [7]. Previous studies have also observed the anti-inflammatory effects of rTM in mice [6,8]. The use of rTM treatment may therefore improve outcomes in patients with ARDS and DIC by controlling the coagulation system and suppressing inflammation. However, the clinical effects of rTM treatment in these patients have yet to be examined. Here, we conduct a retrospective multicenter cohort study of the influence of rTM treatment on in-hospital mortality in patients with both ARDS and DIC.
Materials and Method
Study design and data source
We conducted a retrospective analysis of patients with ARDS and DIC who had been admitted to the intensive care units (ICUs) of 3 university hospitals in the Kansai region of Japan between March 1, 2008 and February 29, 2016. All data were extracted from clinical records. This study was approved by the institutional ethics committee of Kansai Medical University Hospital (Approval number: H120721)
Patient selection
We first identified patients who had been diagnosed with both ARDS and DIC during the study period. ARDS was diagnosed according to the Berlin definition [9]. The Japanese Association for Acute Medicine has developed a DIC score for diagnostic purposes and patients with a score of 4 or more were identified as having DIC. Potential subjects were selected according to the following criteria: patients aged 20 years or older who were admitted to the ICUs of the participant hospitals, patients who were diagnosed as having both ARDS and DIC and patients who were administered rTM every day for 6 days after ICU admission. We excluded patients who were diagnosed with diseases other than ARDS and DIC, diagnosed with hepatic failure or hematological malignancy, did not receive rTM treatment from the first day of ICU admission, or had been administered rTM for fewer than 6 days.
The subjects were divided into 2 groups: A control group comprising patients who were not administered rTM and an rTM group comprising patients who were administered rTM. The decision to administer rTM in each patient was made by their attending physician without randomizing. The dose of rTM was set at 380 U/kg for patients with a minimum estimated glomerular filtration rate (eGFR) of 30 mL/min, or 130 U/kg for patients with an eGFR below 30 mL/min. The rTM administration duration was 6 days (or until the patient died, whichever was earlier) and administration was stopped at the discretion of the treating physician.
Patient characteristics
We collected information on the following patient baseline characteristics: Age, sex, source of sepsis (lung, abdomen, others and no sepsis), severity of ARDS using the Berlin definition, Acute Physiology and Chronic Health Evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score and DIC score.
Data were also obtained on the duration of mechanical ventilation, maximum peak inspiratory pressure, minimum PaO2/fraction of inspiratory oxygen (P/F) ratio, utilization of antithrombin (AT) concentrate and low-dose steroid administration (hydrocortisone sodium phosphate at ≤ 200 mg/day or methylprednisolone at ≤ 2.5 mg/kg/day) in each patient.
Outcome measures
The main outcome measure used in this study was in-hospital mortality. The secondary outcome measures were the platelet count component of the SOFA score, the respiratory component of the SOFA score, the DIC score and the P/F ratio for a week after ICU admission. In the platelet count component of the SOFA score, a coagulation sub-score is given to each patient based on platelet count thresholds. This sub-score ranges from 0 (platelet count: ≥ 150 × 103/μl) to 4 (platelet count: <20 × 103/μl) and the raw platelet count is not included in the SOFA score.
Statistical Analysis
Continuous variables were calculated as means and standard deviations and categorical variables were calculated as percentages. We used Student’s t-test or Welch’s t-test following Levene’s test to compare continuous variables between the 2 groups. Categorical variables were analyzed using the Chi-square test or Fisher’s exact test. The various scores were calculated as median values accompanied by the interquartile range and were analyzed using the Mann-Whitney U test. Univariate analyses were used to identify patient characteristics that were significantly different between the survivors and non-survivors. These factors would then be included as covariates in a Cox proportional hazards regression model with in-hospital mortality as the dependent variable; the main independent variable of interest was the use of rTM. In addition, we also conducted a sensitivity analysis that included other independent variables with P values below 0.2 in the univariate analyses. The sensitivity analysis was conducted for all patients as well as a subgroup comprising only septic patients. The hazard ratios (HRs) for the independent variables were calculated. The Mantel-Haenszel test was used to analyze in-hospital mortality associated with rTM use among the different levels of ARDS severity.
The differences in changes in the DIC score and the platelet count component of the SOFA score between the control group and rTM group were analyzed using two-way repeated measures analysis of variance.
P values lower than 0.05 were regarded as statistically significant. All analyses were performed using SPSS Version 24.0 (IBM Japan, Ltd., Tokyo, Japan).
Results
The control group and rTM group comprised 38 patients and 37 patients, respectively (Table 1). There were 36 survivors and 39 non-survivors (Table 2). There were no statistically significant differences in patient characteristics between the control group and rTM group, as well as between the survivors and non-survivors. The variables of with P values below 0.2 were age (P=0.15), source of sepsis (P=0.17), rTM use (P=0.02) and AT concentrate use (P=0.17) between the survivors and non-survivors. The most common level of ARDS severity in the control group and rTM group was moderate ARDS (47.4% and 56.8%, respectively), followed by mild ARDS (31.6% and 27.0%, respectively) and severe ARDS (21.1% and 16.2%, respectively); there was no significant difference among these severity levels (P=0.71). There were also no significant differences in the in-hospital medical care provided to the control group and rTM group, where the minimum P/F ratios within 24 h of ICU admission were 167.4 ± 66.9 mm Hg and 160.4 ± 61.2 mm Hg, respectively (P=0.64).
|
Variables
|
Control
(n=38) |
rTM
(n=37) |
P value |
|
Number of patients
|
|
|
|
|
Hospital A
|
21 |
16 |
|
|
Hospital B
|
15 |
15 |
|
|
Hospital C
|
2 |
6 |
|
| Patient characteristics |
|
|
|
| Age (years) |
69.7 ± 11.5 |
68.5 ± 17.1 |
0.72 |
| Male (%) |
63.2 |
81.1 |
0.08 |
| Source of sepsis (%) |
|
|
|
| Lung |
57.9 |
67.6 |
0.46 |
| Abdomen |
28.9 |
13.5 |
| Others |
7.9 |
13.5 |
| No sepsis |
5.3 |
5.4 |
| ARDS severity (%) |
|
|
|
| Mild |
31.6 |
27.0 |
0.71 |
| Moderate |
47.4 |
56.8 |
| Severe |
21.1 |
16.2 |
| APACHE II score |
25.8 ± 6.2 |
27.4 ± 6.1 |
0.26 |
| SOFA score (IQR) |
13.0 (10.0-14.3) |
12.0 (11.0-14.0) |
0.84 |
| DIC score (%) |
|
|
|
| 4 |
31.6 |
32.4 |
0.60 |
| 5 |
31.6 |
21.6 |
| 6 |
21.1 |
24.3 |
| 7 |
2.6 |
10.8 |
| 8 |
13.2 |
10.8 |
| Year |
|
|
|
| 2008 |
12 |
0 |
<0.001 |
| 2009 |
11 |
0 |
| 2010 |
0 |
1 |
| 2011 |
2 |
3 |
| 2012 |
1 |
3 |
| 2013 |
1 |
12 |
| 2014 |
5 |
11 |
| 2015 |
5 |
6 |
| 2016 |
1 |
1 |
| Medical care |
|
|
|
| Duration of MV (days) |
25.9 ± 27.0 |
19.7 ± 19.4 |
0.26 |
Maximum PIP
during MV (cm H2O) |
28.5 ± 6.4 |
27.2 ± 5.2 |
0.36 |
| Minimum P/F ratio within 24 h of ICU admission |
167.4 ± 66.9 |
160.4 ± 61.2 |
0.64 |
| AT concentrate use (%) |
71.1 |
75.7 |
0.65 |
| Steroid use (%) |
39.5 |
48.6 |
0.42 |
Table 1: Patient characteristics and outcomes in the control and rTM groups (n=75).
| Variables |
Survivors
(n=36) |
Non-survivors
(n=39) |
P value |
| Number of patients |
|
|
|
| Hospital A |
20 |
17 |
0.25 |
| Hospital B |
11 |
19 |
| Hospital C |
5 |
3 |
| Patient characteristics |
|
|
|
| Age (years) |
66.6 ± 17.7 |
71.4 ± 10.4 |
0.15 |
| Male (%) |
77.8 |
66.7 |
0.28 |
| Source of sepsis (%) |
|
|
|
| Lung |
52.8 |
71.8 |
0.17 |
| Abdomen |
30.6 |
12.8 |
| Others |
11.1 |
10.3 |
| No sepsis |
5.6 |
5.1 |
| ARDS severity (%) |
|
|
|
| Mild |
30.6 |
28.2 |
0.26 |
| Moderate |
58.3 |
46.2 |
| Severe |
11.1 |
25.6 |
| APACHE II score |
26.0 ± 5.2 |
27.2 ± 6.9 |
0.39 |
| SOFA score (IQR) |
11.5 (9.3-14.8) |
13.0 (11.0-14.0) |
0.24 |
| DIC score (IQR) |
5.0 (4.0-6.0) |
5.0 (4.0-6.0) |
0.57 |
| Year |
|
|
|
| 2008 |
4 |
8 |
0.32 |
| 2009 |
6 |
5 |
| 2010 |
1 |
0 |
| 2011 |
2 |
3 |
| 2012 |
1 |
3 |
| 2013 |
8 |
5 |
| 2014 |
8 |
8 |
| 2015 |
5 |
6 |
| 2016 |
1 |
1 |
| Medical care |
|
|
|
| Duration of MV (days) |
20.2 ± 23.1 |
25.3 ± 24.2 |
0.35 |
Maximum PIP
during MV (cm H2O) |
27.4 ± 4.6 |
28.2 ± 6.9 |
0.54 |
| Minimum P/F ratio within 24 h of ICU admission |
170.0 ± 57.7 |
158.4 ± 69.2 |
0.43 |
| AT concentrate use (%) |
80.6 |
66.7 |
0.17 |
| Steroid use (%) |
47.2 |
41.0 |
0.59 |
| rTM use (%) |
63.9 |
35.9 |
0.02 |
Values are presented as mean ± standard deviation for continuous variables and number (percentage) or score (IQR) for categorical variables
APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: Acute Respiratory Distress Syndrome; AT: Antithrombin; DIC: Disseminated Intravascular Coagulation; ICU: Intensive Care Unit; IQR: Interquartile Range; MV: Mechanical Ventilation; P/F: PaO2/Fraction of Inspiratory Oxygen; PIP: Peak Inspiratory Pressure; rTM: Recombinant Human Soluble Thrombomodulin; SOFA: Sequential Organ Failure Assessment .
Table 2: Patient characteristics and outcomes in the survivors and non-survivors (n=75).
The clinical outcomes according to rTM use and ARDS severity are presented in Table 3. The length of ICU stay was significantly shorter (P=0.05) in the rTM group (15.6 ± 7.2 days) when compared with the control group (20.8 ± 14.4 days). In addition, in-hospital mortality was significantly lower (P=0.02) in the rTM group (37.8%) than in the control group (65.8%). Similarly, cumulative 90 day mortality was significantly lower (P=0.03) in the rTM group (42.8%) than in the control group (76.0%). ARDS severity was not associated with the clinical outcomes. In addition, there were no significant differences in survival among the different levels of ARDS severity (Figure 1).
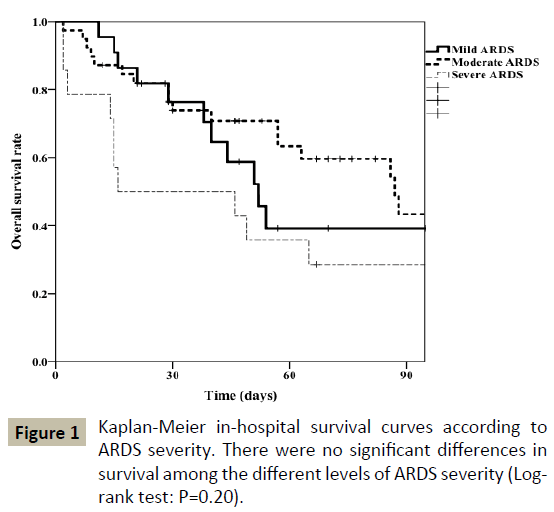
Figure 1: Kaplan-Meier in-hospital survival curves according to ARDS severity. There were no significant differences in survival among the different levels of ARDS severity (Logrank test: P=0.20).
|
Variables
|
rTM use |
P value |
ARDS severity |
P value |
Control
(n=38) |
rTM
(n=37) |
Mild
(n=22) |
Moderate
(n=39) |
Severe
(n=14) |
| ICU stay (days) |
20.8 ± 14.4 |
15.6 ± 7.2 |
0.05 |
21.2 ± 11.7 |
16.6 ± 8.5 |
18.1 ± 17.6 |
0.16 |
| Hospital stay (days) |
54.0 ± 59.6 |
68.0 ± 72.1 |
0.36 |
50.2 ± 36.6 |
73.2 ± 82.9 |
43.4 ± 39.9 |
0.19 |
| In-hospital mortality (%) |
65.8 |
37.8 |
0.02 |
50.0 |
46.2 |
71.4 |
0.26 |
| Cumulative 90 day mortality (%) |
76.0 |
42.8 |
0.03 |
60.8 |
62.1 |
71.4 |
0.20 |
Table 3 Clinical outcomes according to rTM use and ARDS severity (n=75).
| ARDS severity |
rTM use |
In-hospital mortality (%) |
P value |
| Mild (n=22) |
Control |
75.0 |
0.04 |
| rTM |
20.0 |
| Moderate (n=39) |
Control |
55.6 |
| rTM |
38.1 |
| Severe (n=14) |
Control |
75.0 |
| rTM |
66.7 |
Table 4: Association between rTM use and in-hospital mortality stratified by ARDS severity (n=75).
Table 4 shows the in-hospital mortality in the control group and rTM group according to ARDS severity. In all severity levels, the rTM group had lower in-hospital mortality than the control group. Overall, rTM use was significantly associated with inhospital mortality regardless of ARDS severity (Mantel-Haenszel test: P=0.04).
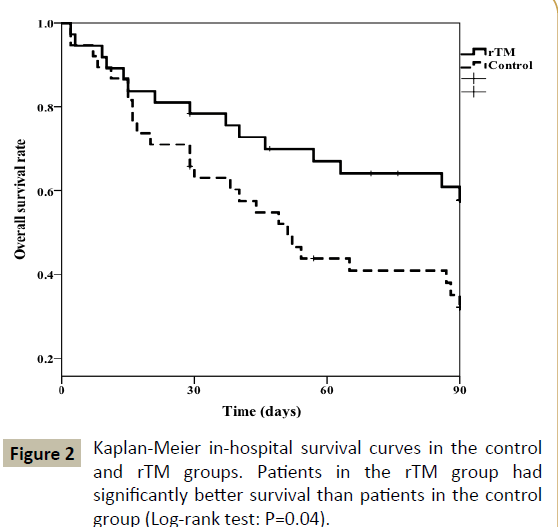
Figure 2: Kaplan-Meier in-hospital survival curves in the control and rTM groups. Patients in the rTM group had significantly better survival than patients in the control group (Log-rank test: P=0.04).
The Kaplan-Meier curves of both groups are shown in Figure 2. The rTM group had significantly better survival than the control group according to the log-rank test (P=0.04). As none of the patient characteristics were significantly associated with mortality, the Cox proportional hazards regression analysis only included the use of rTM as the independent variable. As shown in Table 5, rTM use was significantly associated with a reduction in crude in-hospital mortality (HR: 0.49; 95% confidence interval: 0.26-0.95; P=0.03). This association did not lose significance even after the addition of the sources of sepsis, AT concentrate use and age as covariates in the sensitivity analysis of all patients (Table 5). In septic patients, the HR of rTM use was 0.31 (95% confidence interval: 0.10-0.92; P=0.04).
|
Variables
|
Hazard ratio |
95% CI |
P value |
| Independent variables: rTM use, source of sepsis, AT concentrate use and age in all patients (n=75) |
| rTM use |
0.23 |
0.08-0.66 |
<0.01 |
| Source of sepsis (Ref: No sepsis) |
|
|
0.15 |
| Lung |
2.20 |
0.27-18.2 |
0.46 |
| Abdomen |
0.45 |
0.04-4.70 |
0.51 |
| Others |
2.14 |
0.16-28.85 |
0.57 |
| AT concentrate use |
0.56 |
0.17-1.83 |
0.34 |
| Age |
1.03 |
0.99-1.07 |
0.15 |
|
Independent variables: rTM use, source of sepsis, AT concentrate use and age in septic patients (n=70)
|
| rTM use |
0.31 |
0.10-0.92 |
0.04 |
| Source of sepsis (Ref: Lung) |
|
|
0.12 |
| Abdomen |
0.24 |
0.06-0.96 |
0.04 |
| Others |
1.05 |
0.19-5.75 |
0.95 |
| AT concentrate use |
0.38 |
0.10-1.35 |
0.13 |
| Age |
1.04 |
0.99-1.08 |
0.10 |
AT: Antithrombin; CI: Confidence Intervals; rTM: Recombinant Human Soluble Thrombomodulin.
Table 5 Results of Cox hazard regression analyses of in-hospital mortality (n=75).
The two-way repeated measures analysis of variance detected significant differences between the 2 groups in changes in the platelet count component of the SOFA score (P<0.001; Figure 3a) and the DIC score (P<0.01; Figure 3b). The rTM group had a significantly higher platelet count component of the SOFA score on Day 5 (P<0.01) and DIC score on Day 4 (P=0.04); however, no statistical differences were observed in these variables during the other days. Although there was no significant difference in P/F ratio within 24 h of ICU admission between the 2 groups, the P/F ratio was significantly higher throughout the 7-day period after ICU admission in the rTM group (P<0.01; Table 6). In particular, the rTM group had a significantly higher P/F ratio on Day 7 (P=0.04). In septic patients, the platelet count component of the SOFA score (P<0.001), the DIC score (P=0.01) and P/F ratio (P<0.001) throughout the 7-day period after ICU admission were also higher in the rTM group relative to the control group (data not shown).
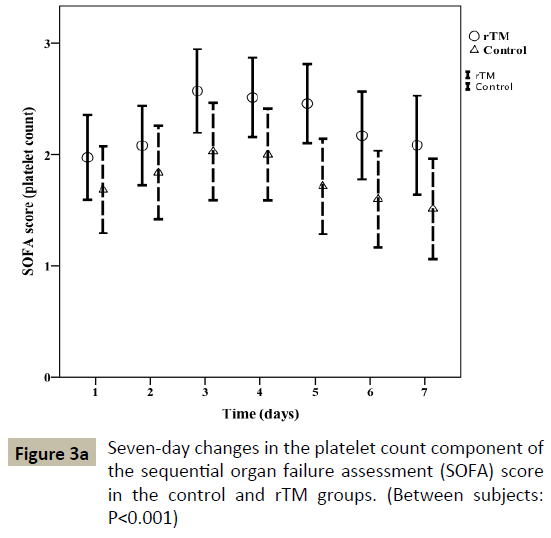
Figure 3a: Seven-day changes in the platelet count component of the sequential organ failure assessment (SOFA) score in the control and rTM groups. (Between subjects: P<0.001)
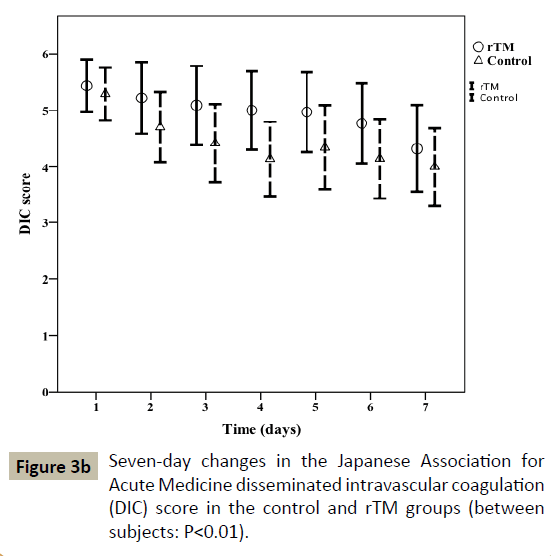
Figure 3b: Seven-day changes in the Japanese Association for Acute Medicine disseminated intravascular coagulation (DIC) score in the control and rTM groups (between subjects: P<0.01).
| Variables |
Control
(n=38) |
rTM
(n=37) |
P value |
| P/F ratio |
|
|
|
| Day 1 |
167.4 ± 66.9 |
160.4 ± 61.2 |
0.01 |
| Day 2 |
215.0 ± 90.8 |
232.3 ± 92.3 |
| Day 3 |
240.3 ± 96.0 |
263.1 ± 111.9 |
| Day 4 |
249.9 ± 97.6 |
284.2 ± 104.1 |
| Day 5 |
264.5 ± 122.1 |
294.3 ± 110.7 |
| Day 6 |
250.3 ± 104.0 |
286.3 ± 114.9 |
| Day 7 |
248.2 ± 110.1 |
304.9 ± 106.2 |
Values are presented as mean ± standard deviation.
P/F: PaO2/Fraction of Inspiratory Oxygen; rTM: Recombinant Human Soluble Thrombomodulin.
Table 6: P/F ratios throughout the 7-day period after ICU admission (n=75).
Discussion
This study is, to the best of our knowledge, the first to examine the influence of daily rTM use on in-hospital mortality in patients with both ARDS and DIC. Our results indicate that rTM administration was associated with reductions in in-hospital mortality. The results were similar in the subgroup of patients who had sepsis-induced ARDS.
In an analysis of acute exacerbation of idiopathic pulmonary fibrosis, Isshiki et al. compared outcomes in patients treated with rTM and conventional therapy [10]. That study found that patients treated with rTM had better survival over 90 days. Despite the difference in target diseases between their study subjects and ours, the similar findings are noteworthy. Another study reported that rTM treatment improved lung injury scores and 90 day mortality in patients with sepsis-induced ARDS and DIC [11]. Their findings corroborate those of our sub analysis of similar patients who experienced improvements in oxygenation function and 90-day mortality.
A previous retrospective analysis found that patients with ARDS and DIC experienced improved P/F ratios after 7 days of rTM administration [12]. Similarly, Ogawa et al. showed that rTM administration improved respiratory function (using the lung injury score and the respiratory component of the SOFA score) in Japanese patients with sepsis [11]. However, our study is the first to continuously monitor P/F ratios for 7 days after ICU admission in patients with ARDS and DIC. Although the patients in Isshiki et al. had substantially higher P/F ratios [10] than our subjects, both studies observed similar improvements after rTM treatment. Furthermore, previous studies have indicated that higher oxygenation is associated with improved prognoses [13,14]. As shown in Table 6, the rTM group in our study generally experienced higher levels of oxygenation within 7 days of starting rTM treatment. Additionally, our analysis did not detect any improvements in DIC score and the platelet count component of the SOFA score. It is therefore possible that the improvement in oxygenation function contributed to the improvement in clinical outcomes. However, it is unclear if the improvements are due to increased oxygenation function following the inhibition of microthrombi formation or if they are attributable to other mechanisms that are presently unknown. The mechanisms should be examined in future studies.
Although our study did not examine how rTM administration improves prognoses in patients with ARDS and DIC, a possible mechanism is the lowering of HMGB-1 levels. A previous study reported that HMGB-1 levels in the lungs of ARDS mice were higher than in non-ARDS mice and that rTM administration prolonged survival time and halted the exacerbation of ARDS [15]. In addition, rTM treatment has been reported to prevent lipopolysaccharide-induced pulmonary vascular injury through protein C activation in rats [16,17], as well as increase HMGB-1 concentrations in patients with acute lung injury [18]. In ARDS patients who were administered rTM, survivors had significantly lower HMGB-1 levels than non-survivors after 7 days of treatment [19]. While our study did not measure HMGB-1 concentrations, our findings that rTM treatment improves prognoses in ARDS patients do not contradict the results of these previous studies.
The findings of this study should be considered in the context of several limitations. Firstly, this was a retrospective study with a relatively small sample size. Secondly, there may be therapeutic biases that affected the results of the time course of DIC score and the platelet count component of the SOFA core. Thirdly, there may be confounding factors that were not included in analysis. For example, the analysis did not account for differences in underlying disease. Severe diseases such as chronic obstructive pulmonary disease or chronic kidney failure would affect patient prognosis. In addition, as the management of ARDS may have improved over time, it is possible that this and other unidentified confounding factors had influenced in-hospital mortality during the relatively long study period. For example, the determination of ARDS severity was based on the P/F ratio and the initial positive end-expiratory pressure setting for mechanical ventilation may vary among the hospitals and physicians. The imprecise identification of moderate ARDS cases as mild ARDS cases may have resulted in disproportionately high in-hospital and cumulative 90-day mortality rates in patients with mild ARDS, thereby causing a lack of significant associations between these outcomes and ARDS severity. Fourthly, we did not collect raw data on platelet count and C-reactive protein levels in the initial protocol of this study. As these variables are useful for understanding the clinical course of ARDS and DIC, they should also be included in downstream analyses. Finally, we calculated that the detection of an in-hospital mortality reduction of 10% would indicate a statistical power of 0.36; similarly, the detection of an in-hospital mortality reduction of 20% would indicate a statistical power of 0.09. There would therefore need to be at least 120 cases in each group to detect a reduction of 10% in in-hospital mortality. Accordingly, there is a need to further increase the number of cases to compare mortality rates.
Conclusion
Our study indicates that rTM treatment was able to significantly improve prognoses in patients with both ARDS and DIC. However, there is a need to confirm these findings in large-scale prospective studies, including randomized control trials. Our analysis suggests that rTM treatment improved oxygenation function relatively quickly after administration and this mechanism warrants further examination.
Acknowledgement
We thank the patients and their families for their participation in the study.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the internal ethics review board of Kansai Medical University Hospital (Approval Number: H120721).
Consent for publication
Informed consent was obtained from all enrolled patients or their relatives.
Availability of data and material
The dataset supporting the conclusions of this article is available upon request. Please contact the corresponding author (Takeshi Umegaki) for data requests.
Competing interests
The authors declare that they have no competing interests.
References
- Bone RC, Francis PB, Pierce AK (1976) Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med 61: 585-859.
- Gando S, Kameue T, Matsuda N, Sawamura A, Hayakawa M, et al. (2004) Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: Role of neutrophil and endothelial activation. Inflammation 28: 237-244.
- Laterre PF, Wittebole X, Dhainaut JF (2003) Anticoagulant therapy in acute lung injury. Crit Care Med 31: S329-S336.
- Esmon CT, Owen WG (1981) Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A 78: 2249-2252.
- Esmon CT (2005) The interactions between inflammation and coagulation. Br J Haematol 131: 417-430.
- Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, et al. (2005) The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein; A novel anti-inflammatory mechanism. J Clin Invest 115: 1267-1274.
- Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, et al. (2007) Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: Results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 5: 31-41.
- Takahashi Y, Matsutani N, Dejima H, Nakayama T, Okamura R, et al. (2016) Therapeutic potential of recombinant thrombomodulin for lung injury after pneumonectomy via inhibition of high-mobility group box 1 in mice. J Trauma Acute Care Surg 81: 868-875.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, et al. (2012) Acute respiratory distress syndrome: The Berlin definition. JAMA 307: 2526-2533.
- Isshiki T, Sakamoto S, Kinoshita A, Sugino K, Kurosaki A, et al. (2015) Recombinant human soluble thrombomodulin treatment for acute exacerbation of idiopathic pulmonary fibrosis: A retrospective study. Respiration 89: 201-207.
- Ogawa Y, Yamakawa K, Ogura H, Kiguchi T, Mohri T, et al. (2012) Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Surg 72: 1150-1157.
- Miyoshi S, Ito R, Katayama H, Dote K, Aibiki M, et al. (2014) Combination therapy with sivelestat and recombinant human soluble thrombomodulin for ARDS and DIC patients. Drug Des Devel Ther 8: 1211-1219.
- Bone RC, Maunder R, Slotman G, Silverman H, Hyers TM, et al. (1989) An early test of survival in patients with the adult respiratory distress syndrome. The PaO2/FiO2 ratio and its differential response to conventional therapy. Prostaglandin E1 Study Group. Chest 96: 849-851.
- Heffner JE, Brown LK, Barbieri CA, Harpel KS, DeLeo J (1995) Prospective validation of an acute respiratory distress syndrome predictive score. Am J Respir Crit Care Med 152: 1518-1526.
- Kudo D, Toyama M, Aoyagi T, Akahori Y, Yamamoto H, et al. (2013) Involvement of high mobility group box 1 and the therapeutic effect of recombinant thrombomodulin in a mouse model of severe acute respiratory distress syndrome. Clin Exp Immunol 173: 276-287.
- Uchiba M, Okajima K, Murakami K, Nawa K, Okabe H, et al. (1995) Recombinant human soluble thrombomodulin reduces endotoxin-induced pulmonary vascular injury via protein C activation in rats. Thromb Haemost 74: 1265-1270.
- Uchiba M, Okajima K, Murakami K, Johno M, Okabe H, et al. (1996) Recombinant thrombomodulin prevents endotoxin-induced lung injury in rats by inhibiting leukocyte activation. Am J Physiol 271: 470-475.
- Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, et al. (2004) Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170: 1310-1316.
- Tsushima K, Yokoyama T, Koizumi T, Kubo K, Tatsumi K (2013) The concept study of recombinant human soluble thrombomodulin in patients with acute respiratory distress syndrome. Int J Clin Med 4: 488-495.