- (2016) Volume 0, Issue 0
Lea Matsuoka*, Alejandro Pita, Dilip Parekh
Department of Surgery, Keck School of Medicine at the University of Southern California, USA
Received December 2nd, 2015 - Accepted December 30th, 2015
Pancreatic enucleation has been performed for small, benign or premalignant lesions of the pancreas. The goal of this parenchymapreserving strategy is to reduce the risk of exocrine and endocrine insufficiency and potentially the risks associated with pancreatic and biliary anastomoses. Studies have shown open pancreatic enucleation to be a viable option for patients, with a debatable increased risk of pancreatic fistula. With the advent of minimally invasive techniques and increasing experience, centers have started to perform laparoscopic pancreatic enucleation. Small studies have been performed demonstrating the safety and feasibility of laparoscopic pancreatic enucleation, with patient positioning and port placement dependent upon the location of the lesion. Laparoscopic intraoperative ultrasound plays an important role in the localization of these tumors and their proximity to the pancreatic duct and vascular structures, which helps to determine appropriateness for enucleation.
Laparoscopy; Pancreas
IOUS intraoperative ultrasound
Pancreatic resections are complex operations that result in a high incidence of postoperative complications, including pancreatic endocrine and exocrine insufficiency. Parencyhma-preserving procedures such as enucleation have the potential to decrease the complications associated with anastomoses and decrease the rate of pancreatic insufficiency. Enucleations have been performed for small, benign or premalignant lesions such as cystic tumors, neuroendocrine tumors and IPMN. Cauley et al. described 45 consecutive patients who underwent pancreatic enucleation and compared them to a matched cohort of 90 patients who had undergone formal resection [1]. Average tumor size was 2.3 cm, with 56% of lesions in the body/ tail and the rest in the uncinate process/head/neck. These tumors included neuroendocrine (47%), mucinous cystic tumors (22%), serous/simple cysts (22%) and other benign lesions (9%). The patients undergoing enucleation had statistically significantly shorter operative times, blood loss and intensive care unit days. The enucleation group also had less serious morbidities and a lower rate of endocrine and exocrine insufficiency. Additional studies comparing enucleation and standard pancreatic resections have also reported shorter operative times, lower blood loss and shorter hospital and/or intensive care unit days [1-4].
The Achilles’ heel of pancreatic surgery is postoperative pancreatic fistula. Cauley et al. reported no difference in the incidence of pancreatic fistulas following enucleation compared to standard pancreatic resections in their matched cohort of patients, whereas Pitt et al. and Hackert et al. reported statistically significant higher rates of pancreatic fistula with enucleation procedures compared to standard pancreatic resections [1, 2, 4]. These differences in outcomes may be secondary to the small number of patients in the enucleation cohort and differences in reporting and diagnosing pancreatic fistula.
With the advent of laparoscopic techniques, centers have begun reporting results from laparoscopic enucleation procedures. Many of these studies examined the outcomes following laparoscopic pancreatic procedures as a whole, so pancreatic enucleation was grouped with distal pancreatectomies and other resections [5-7]. Table 1 lists the outcomes from studies specifically describing results from a cohort of patients undergoing laparoscopic enucleations [8-21]. The operative time ranged from 85- 140 minutes, with a 4% mortality rate only in one study (the remaining studies all reported 0% mortality). Conversion rates, pancreatic fistula rates and length of stays ranged widely. This is again likely because of the small number of patients in each study, as well as the varying definitions used for pancreatic fistula.
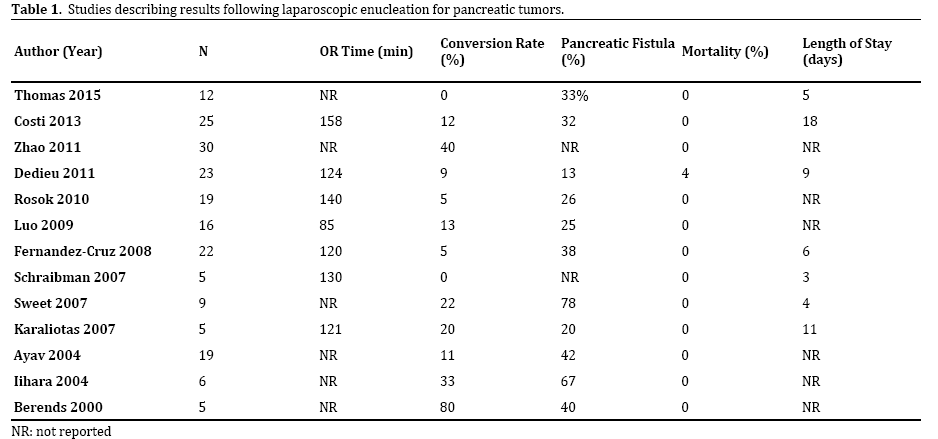
Our recent study published in 2015 consisted of 12 patients who underwent laparoscopic hand-assis4ted pancreatic enucleation for presumed side-branch IPMN [21]. The study also included an additional 5 patients who underwent laparoscopic hand-assisted pancreatic head resection. Our average length of stay for the enucleation group was 5 days with pancreatic fistulas occurring in 4 of the patients (only one required additional drain placement). Our follow-up was 2.8 years. We support the miduse of pancreatic enucleation for benign and premalignant tumors such as side-branch IPMN, as operative outcomes are acceptable and post-operative recurrence risk is low. The largest study by Zhao et al. was published in 2011 and included 30 patients who underwent laparoscopic enucleation [22]. Their study included 292 patients who underwent surgical treatment of insulinoma. The majority of patients underwent enucleation; 199 patients underwent open enucleation and 30 patients underwent attempted laparoscopic enucleation. The study reported no difference in blood loss, operative times, complications or lengths of stay between the open and laparoscopic group, but unfortunately their groups also included patients who underwent procedures other than enucleation, so definitive conclusions regarding laparoscopic versus open enucleation cannot be made. The only other study reporting a comparison between laparoscopic and open enucleation was a small study published in 2009 by Karaliotas et al. who retrospectively reviewed the charts for seven patients undergoing open enucleation for insulinoma and five patients undergoing laparoscopic enucleation for insulinoma [16]. The open enucleation patients had an increased length of stay (14 days vs. 11 days) and similar rates of pancreatic fistula (29% vs. 20%) compared to the laparoscopic enucleation group.
The studies published on laparoscopic enucleation support the safety and feasibility of laparoscopic enucleation for small, benign or premalignant pancreatic tumors. Despite the lack of literature directly comparing laparoscopic and open enucleation, the published results compare favorably with those following open pancreatic enucleation.
Laparoscopic Enucleation Surgical Techniques
Patient positioning on the operating table and exact trocar position are dictated by the preoperative anatomical location of the tumor. Enucleation is generally indicated when the lesion is solitary and does not involve adjacent important structures, such as the pancreatic duct or splenic vein.
Tumors in the Posterior Head of the Pancreas: The patient is placed in the left lateral position with adequate flexion of the operating table to maximize the space between the iliac crest and the right inferior costal margin [5, 17, 23] (Figure 1). A 10 mm trocar is inserted in the midclavicular line 10 cm below the costal margin. Three additional working ports are introduced in a crescent shape in the right upper quadrant, as illustrated in Figure 2. After mobilization of the hepatic flexure of the colon, additional retractors may be placed as necessary in the posterior axillary line and the mid-axillary line just below the costal margin to provide retraction of the kidney and the right hepatic lobe. The Kocher maneuver is then performed. This maneuver provides adequate exposure of the posterior pancreatic head and uncinate process, as well as the aorta and inferior vena cava. Hook or endoshear electrocautery in combination with blunt dissection is then used to enucleate the tumor from the surrounding parenchyma, while vessels are secured with clips. Alternatively, an ultrasonic scalpel or bipolar vesselsealing device can be used. After removal of the lesion, careful examination of the tumor bed is performed, and a drain is left in place in the retropancreatic space.
An anterior approach through the gastrocolic ligament may also be utilized in combination with extensive kocherization. Alternatively, for a hand-assisted approach, a hand-port may be placed in the right subcostal area, with placement of two trocars 2 cm above and medial and lateral to the umbilicus.
Tumors in the Anterior Head of the Pancreas: The patient is positioned in the left semi-lateral position and four 10-12 mm trocars are placed [10, 24]: 3-4 cm above the umbilicus, subxiphoid, and subcostally on the miduse axillary and midclavicular lines (Figure 3). The gastrocolic ligament is then divided to create a window large enough to allow for inspection from beyond the GDA to the splenic hilum. This is followed by dissection of the avascular plane between the antrum/pyloric region of the stomach and the anterior pancreatic head. For pancreatic tumors located in the superior edge of the pancreatic head, separation of the common hepatic artery from the superior pancreatic border is performed. When the tumor is located along the inferior margin of the head, the inferior head border is separated from the superior mesenteric vein/portal vein by clipping and dividing the small collaterals vessels. Enucleation is performed as previously described. During the process of enucleation, lifting of the tumor from its bed facilitates the dissection. Once again, after the excision of the lesion, the tumor bed is carefully examined and a drain is left in place.
As mentioned for tumors of the posterior pancreatic head, a hand-port may be placed in the right subcostal area, with placement of two trocars 2 cm above and medial and lateral to the umbilicus.
Tumors in the Body and Tail of the Pancreas: Lesions in the pancreatic tail and distal body can be approached with the patient arranged in the supine modified lithotomy, right lateral, or half-decubitus position, based on the exact tumor location and the preference of the operating surgeon [11, 19, 24, 25]. Four trocars are placed, as seen in Figure 4. The lesser sac is entered by dividing the gastrocolic ligament along with the short gastric vessels. Exposure of the pancreas in its entire length is obtained, and separation of the avascular plane between the stomach and anterior pancreatic capsule is performed. The splenic flexure of the colon is then mobilized and the inferior pancreatic peritoneum is divided. After finding the appropriate plane between the tumor capsule and surrounding pancreatic parenchyma, the tumor is enucleated in a similar fashion as previously described. For posterior tumors, lifting of the inferior margin of the pancreatic body (previously released from its peritoneal attachments) is performed and the posterior pancreatic surface is mobilized. If the tumor is in close proximity to the splenic vein, careful dissection to isolate the vein is performed. Inspection of the tumor bed with placement of a drain follows.
For tumors located in the pancreatic body and tail, a hand-port can be placed in the upper midline. Two trocars are inserted along a line 2 cm above the umbilicus, depending on the exact location of the lesion.
Role of Laparoscopic Intraoperative Ultrasound
Pancreatic lesions are localized using a combination of techniques, including preoperative imaging studies, visual inspection, manual or laparoscopic instrument palpation, and the use of intraoperative ultrasound (IOUS). The use of IOUS has proved to be of key significance in the enucleation of pancreatic tumors. Cystic lesions are easily identified by ultrasound, and neuroendocrine tumors are usually hypoechoic, making it easy to differentiate from the surrounding parenchyma (Figure 5). Series have reported a 60-66% correct tumor location rate achieved with the use of laparoscopic exploration and instrumental palpation alone, which increased to 81-90% when IOUS was performed [17, 20]. IOUS can identify tumors that were not seen on preoperative imaging and can also detect the presence of multiple lesions. Furthermore, in equivocal situations where preoperative images show artifacts or when the tumor is not in the expected location, IOUS plays a crucial role in determining the exact location and characteristics of the tumor.
IOUS not only assists in determining the exact tumor location, but can also delineate the relationship between the lesion and important adjacent structures such as the main pancreatic duct, decreasing the chance of fistula formation [11]. IOUS can facilitate operative decision making between enucleation versus a more extensive resection, as well as for determining whether an anterior or posterior surgical approach should be performed [26]. The lesion should be separated from the pancreatic duct by enough distance to facilitate enucleation without duct disruption, or resection should be considered. Additionally, the proximity of the lesion to important structures such as the splenic, portal, and superior mesenteric vessels is determined to aid in surgical decision making (Figure 5). Whenever available, cooperation with an experienced radiologist in the operating room is recommended.
In the era of minimally invasive techniques for the management of tumors of the pancreas, laparoscopic enucleation has become a valuable alternative to more extensive pancreatic resections. Whenever feasible, this parenchyma-sparing approach not only minimizes the risks of exocrine and endocrine deficiency, but has the potential benefit of decreasing the risks of anastomosisassociated complications and may shorten hospital stay. Localization of the lesion should be performed preoperatively with a combination of appropriate imaging modalities which will be used to decide whether an enucleation or a more extensive pancreatic resection is indicated. The surgical approach and location of ports will generally be dictated by the exact location of the tumor, which should be determined prior to surgical intervention. Invariably, the addition IOUS provides another level of valuable information, such as the relationship between the tumor and adjacent vascular structures, the involvement of the pancreatic duct, and the presence of additional lesions, all of which will influence the decision for the final surgical approach.
The current literature contains small cohorts of patients who have undergone laparoscopic enucleation for pancreatic lesions. These studies support the utility of laparoscopic enucleation for small, benign or premalignant lesions. Further studies need to be performed in order to determine more precisely the risk of pancreatic fistula and the potential benefits related to blood loss, operative time and length of stay.
Authors declare no conflict of interests for this article.