Research Article - (2018) Volume 8, Issue 4
Sani ZM*, Abdullahi IL and Sani A
Biological Sciences Department, Bayero University Kano, Nigeria
Corresponding Author:
Sani ZM
Biological Sciences Department
Bayero University Kano, Nigeria
E-mail: zmsani.bio@buk.edu.ng
Received Date: July 03, 2018; Accepted Date: Aug 06, 2018 Published Date: Aug 15, 2018
Citation: Sani ZM, Abdullahi IL, Sani A (2018) Toxicity Evaluation of Selected Dyes Commonly used for Clothing Materials in Urban Kano, Nigeria. Eur Exp Biol Vol. 8 No. 4:26. doi:10.21767/2248-9215.100067
Copyright: © 2018 Sani ZM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The study was focused on four selected commercial dyes widely used in urban Kano to re-dye fabrics. The dyes were defined by the colours, blue, red, orange and yellow. The aim was to assess and evaluate the concentration of dangerous metallic compounds in the dyes and subsequent toxicity of these compounds in experimental animals (brine shrimps, Artemia salina). The results showed that the concentration levels of cadmium, chromium, lead, copper and zinc varies among the dyes, however, the concentration exceeded permissible limits set by standard authorities such as FEPA (1991) and DOE (2008). The LC50 of the various dyes on the test animals indicated that the blue dye was the most toxic, followed by yellow, red and orange respectively. This finding highlights public health and environmental implication because of direct contact of these dyes with tissue systems of individuals involved in the re-dyeing activity, aquatic organisms, food and other route of administration. Therefore, the use dyes such as those found in this study should be restricted, as they are causing widespread contamination of some areas in urban Kano.
Keywords
Textile dyes; Heavy metals; Toxicity; Lethality test; Brine shrimp (Artemia salina)
Introduction
Dyes are introduced into the environment through industrial effluents from food, drug, cosmetic, textile and dyestuff factories [1-3]. A number of dyes and chemicals used by these textile industries are highly structured polymers and are very difficult to decompose biologically belonging to different groups based on the chemical composition and application. These are the Chromophores (are the nitrogen, carbon, oxygen and sulphur that have alternate single and double bonds) and Auxophores (are the amino, carboxyl, hydroxyl, and sulphonic groups) which are responsible for the various colour patterns and intensity of the textile products [4]. The excessive and indiscriminate use of these dyestuffs has become increasingly a subject of environmental concern.
Due to the complex aromatic structure and stability of these dyes, conventional biological pre-treatment methods are ineffective for degradation [5]. They are thus a potent hazard to the natural sources like soil, water, flora, fauna, livestock and human population. Since large quantities of dyes are used, pollution due to dyes may occur on a significant scale [6]. In Kano, the parts of the environment that subsequently receives these dyes are the land (soils) and aquatic systems (lakes, rivers, ponds, streams, etc). Living organisms are susceptible to the effects of these substances depending on their structure, complexity and metabolic efficiency [7].
Brine shrimp have been used as a “benchtop bioassay” for the discovery and purification of bioactive natural product [8], and they are an excellent choice for elementary toxicity investigations of consumer products. In recent years scientists have been discovering various bioactive compounds with diverse chemical structures, guided by Brine shrimp test a simple and quick bioassay which can be carried out in a chemical laboratory [9]. Brine shrimp, Artemia species, also known as sea monkeys, are marine invertebrates about 1 mm in size. The cysts last for several years and can be hatched without special equipment (directions are given in the supplementary materials). Toxicity testing is carried out by adding different doses of material to small numbers of the shrimp; the entire dose–response is observed for a given period.
Materials and Methods
Collection and preparation of samples
Four dye samples (red, yellow, blue and orange) were obtained from the ‘Kofar Mata’ dyeing pits store in Kano State of Nigeria. The dye samples were prepared for the determination of heavy metals such as cadmium, chromium, lead, copper and zinc in the laboratory.
Determination of heavy metals in dye powder
Heavy metals such as zinc, copper, lead, chromium and cadmium) were determined spectrophotometrically using atomic absorption spectrophotometer (AAS) VGP 210 model as adopted by Ademoroti in his Works [10]. The sample(s) were initially dissolved in the desired solvent for each metal and placed in the flame of the AAS. The isolated metal atoms interact with radiation that has been pre-set to certain wavelengths. The magnitude of the AAS absorption signal being directly proportional to the concentration of the analyte metal in the solution was measured and interpreted.
Brine shrimp lethality test
A number of reliable and very sensitive bioassay techniques which are indicative of toxicity were reported by Fatope [11], which include the one-day duckling bioassay, the chick embryo test, the zebra fish test, test on insect or insect larvae, the rat multipurpose screen and others. Most of these bioassay techniques cannot be used as rapid, general screening procedure for the detection of toxic secondary metabolites because of costs, specifity, sophistication or objections by animal right actives. As such McLaughlin et al. [8], introduced a simple bioassay procedure (Brine shrimp test (BST)) that involves direct isolation of bioactive compounds [12]. It is a simple, rapid, in-house, bench-top and low cost prescreen toxicity test [8].
This research used the Brine shrimp test to check the toxicity of the four selected textile dyes. The species Artemia salina is a widely used experimental animal. It is a crustacean belonging to the family Artemiidae and is commonly found in brackish water. Artemia salina cysts were purchased and maintained at a viable condition in laboratory for the research. The cysts were placed in seawater for 24 hours at room temperature to hatch. After hatching the cysts; extract concentrations of 1000, 100 and 10 μg/ml were prepared with seawater. These concentrations were prepared in triplicate for each sub-sample [13].
Twenty milligrams each of the dye powder were separately dissolved in 2 ml of methanol. From the prepared solutions, Concentrations of 300, 30 and 3 μl were transferred into 5ml capacity vials corresponding to 1000, 100 and 10 μg/ml respectively. All the experimental vials were kept at room temperature for 24 hours for the solvent to evaporate. 2 drops of Dimethyl sulphoxide (DMSO) was added in each vial to enhance the solubility of the extracts, 1 ml of seawater was also added to the vials. Ten brine shrimp larvae were placed into each of the vials and the level of the seawater was adjusted to 3 ml using the control. The control was prepared containing the same volume of seawater, ten brine shrimp larvae but without the extract. The experimental set up was observed for 24 hours to detect and estimate mortality as adopted by Adoum et al. [14]. The Lethal concentration dose (LC50) was obtained by computing relationship between mortality and concentration as expressed by the formula below;
Mmct = Nmm/No. × 100
Where, Mmct – mortality of individuals in time, t (%)
Nmm – average number of dead individuals
No – initial number of living individuals put into each concentration at the start [15].
Results
The result obtained from the heavy metal analysis and brine shrimp toxicity test of the four textile dyes are presented in the Figures 1 and 2, Table 1.
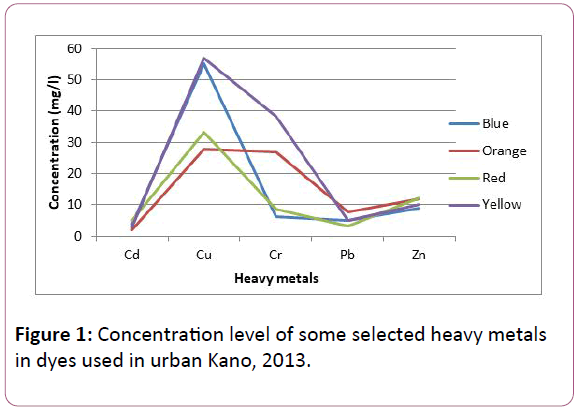
Figure 1: Concentration level of some selected heavy metals in dyes used in urban Kano, 2013.
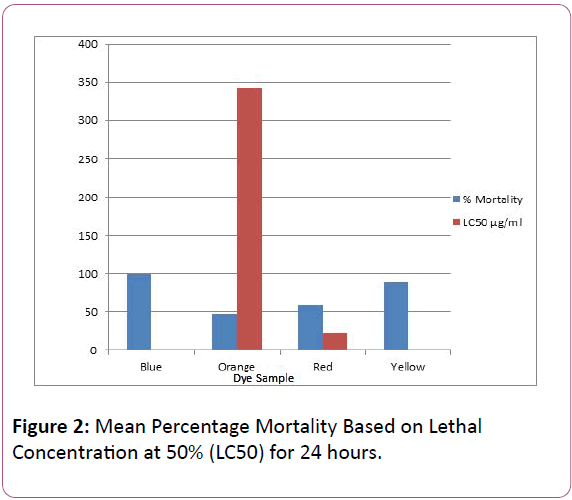
Figure 2: Mean Percentage Mortality Based on Lethal Concentration at 50% (LC50) for 24 hours.
| Dye sample | Concentrations µg/ml | Average Mortality | % Mortality | LC50 µg/ml |
|---|---|---|---|---|
| Blue | 10 | 10 | 100 | 0.00 (0.65-159065.6) |
| 100 | 10 | 100 | ||
| 1000 | 10 | 100 | ||
| Orange | 10 | 3 | 30 | 343.1 (0.00-0.00) |
| 100 | 6 | 60 | ||
| 1000 | 5 | 50 | ||
| Red | 10 | 5 | 50 | 21.5 (124.1-0.00) |
| 100 | 5 | 50 | ||
| 1000 | 8 | 80 | ||
| Yellow | 10 | 8 | 80 | 0.95 (6.40-0.00) |
| 100 | 9 | 90 | ||
| 1000 | 10 | 100 |
Table 1: Percentage mortalitybased on Lethal Concentration at 50%(LC50) for 24 hours.
The Figure 1 shows that cadmium had low concentration in all the dye samples, while copper had a relatively high concentration in all the dye samples.
The Figure 2 shows blue and yellow dyes to be highly toxic having the least LC50 value and highest percentage mortality, then red, with orange being the least toxic having the highest LC50 value.
Discussion
The result for the detection of selected heavy metals in the dyes used in re-dyeing activity in urban Kano is shown in Figure 1. Red dye had the highest mean value for Cadmium and Zinc (5.30 and 12.32 mg/l respectively). Yellow dye had the highest value for Copper and Chromium (57.12 and 38.24 mg/l respective). The heavy metal contents in the dyes exceeded the permissible limit set by FEPA for discharge. This may pose negative impact to the environment when discharged indiscriminately [16] and can also lead to health problems in humans and animals (due to accumulation in their tissues through inhalation, direct contact and ingestion), damage to plants and other forms of life, because most of these dyes are toxic, mutagenic and carcinogenic [17], containing heavy metals and other organic aromatic compounds [18]. Some of the damages these dyes cause include allergic dermatoses and respiratory diseases [19]. Contact dermatitis and asthma were also reported by Thoren et al. [20]. In textile industry workers exposed to dyes to have changes in their immunoglobulin levels [21]. Previous studies have also shown increased risks of colon and rectum cancers which may be related mostly to dyes for synthetic fibres [22,23]. Workers in the textile industry have a twofold risk of contracting bladder cancer compared to workers in other industries such as aviation, agriculture and construction. Several azo dyes have been proven to have genotoxicity when studied with HaCaT cells which are human keratinocytes [24]. The frog embryo teratogenesis assay- Xenopus (FETAX) to establish that some reactive dyes have teratogenic potential.
The result for the brine shrimp lethality test on the dyes (blue, orange, red and yellow dye) commonly used in re-dyeing activity in urban Kano is presented in Figure 2. The result shows blue dye having the least LC50 0.00 μg/ml, with a mean percentage mortality of 100%. The blue and red dyes were found to be more toxic than the yellow dye, the IC50 values being as follows: 392 μg/ml (yellow dye); 370 μg/ml (red dye) and 361 μg/ml (blue dye). This could be due to the concentration of heavy metals in the dye. Yellow dye had an LC50 of 0.95 μg/ml, with a mean percentage mortality of 90%. Red dye had an LC50 of 21.5 μg/ml, with a percentage mortality of 60%. Orange dye being the dye with the highest LC50; 343.1 μg/ml, with a percentage mortality of 47% is said to be the least toxic among the four tested dyes. Majority of textile dyes have LD50 values between 250-2,000 mg/kg body weights, indicating that for a lethal dose many grams of azo dyes have to be consumed in a single dose [24].
Conclusion
In conclusion, the result of the study indicated that textile dyes may be highly toxic due to the presence of high levels of heavy metals, most of which have exceeded the recommended limits. These compounds may pose serious risks to humans, plants and other organisms (both soil and aquatic) through ecological interaction in the ecosystem.
Recommendations
• The use of dyes such as those found in this study should be restricted, as they have caused widespread contamination of some areas in urban Kano.
• Eco-friendly strategies for treatment of dye wastewater before discharge into the environment should be employed.
• Knowlegde on the environmental and health risks of dyes by the users and general public is very limited, thus, there is a need for awareness to the users and general public on the effects of these dyes.