Research Article - (2014) Volume 4, Issue 1
Post Graduate ResearchDepartment of Zoology, St. Albert’s College, Ernakulam, Kochi, Kerala, India
Reactive oxygen species generation and enzymatic and non-enzymatic antioxidant profiling has been emerged as an important area of research due to its correlation with environmental stress where the organism inhabit and as a way to recover from ROS induced damages. Here an attempt has been done to investigate the Glutathione peroxidase activity in four organs viz., gills, liver, kidney, muscle in three species of fish viz., Tialpia (Oreochromis mossambicus), Grey mullet (Mugil cephalus) and Spotted scat (Scatophagus argus) collected from a traditional brackish water farm in Kochi to understand the species-wise and organ-wise alterations in Glutathione peroxidase activity in fish. The results showed a decreasing trend in enzyme activity as liver > gills > Kidney > muscle in all the species of fish selected. In Tilapia (Oreochromis mossambicus) the organ-wise trend was 0.75 ±0.05 μg of GSH consumed/min./mg protein in liver, 0.52±0.03 in gills, 0.34±0.02 in kidney and 0.28±0.009 in muscle. In Grey mullet (Mugil cephalus) glutathione peroxidase activity in liver, gills, kidney and muscles was 3.8±0.2, 3.2±0.2, 1.56±0.09 and 0.99±0.06 μg of GSH consumed/min./mg protein respectively. In Spotted scat (Scatophagus argus) the organwise variation was 2.3±0.09 μg of GSH consumed/min./mg protein in liver, 1.7±0.09 in gills, 0.91±0.006 in kidney and0.66±0.007 in muscle. Hepatic, Branchial, Renal and Muscular Glutathione peroxidase showed a similar decreasing trend in species- wise variation like M. cephalus > S. argus > O. mossambicus.
Glutathione peroxidase, Tilapia, Grey mullet, Spotted scat
Oxygen is absolutely necessary for the life processes, in particular cell respiration. However, the metabolism of oxygen may generate reactive elements called free radicals, in particular the superoxide ion (O2–) and the hydroxyl ion (OH–) [Joanny and Menvielle-Bourg, 2005]. These short-lived and highly reactive oxygen species (ROS) such as O2·- (superoxide), ·OH (hydroxyl radical), and H2O2 (hydrogen peroxide) are continuously generated in vivo. These chemically unstable compounds carry free electrons that react with other molecules, in turn destabilizing them and thereby inducing a chain reaction. In particular, free radicals damage DNA, essential cellular proteins and react with the unsaturated fatty acid of cellular or subcellular membranes. Therefore, they lead to peroxidation of membrane lipids[(Lukaszewicz-Hussain and Moniuszko-Jakoniuk, 2004], which may lead to cell death [Joanny and Menvielle- Bourg, 2005].
In the resting state, the balance between antioxidants and oxidants is sufficient to prevent the disruption of normal physiologic functions [Liocher and Fridovich, 2007; Imlay, 2008]. These antioxidant mechanisms mainly involve specific enzymes (superoxide dismutase or SOD, catalase, gluthation peroxidase or Gpx) as well as radical scavengers that trap free radicals ((antioxidant vitamins A, C, E), thiols and ß-carotene) [Vouldoukis et. al., 2004]. Either increases in oxidants or decreases in antioxidants can disrupt this balance giving rise to elevated levels of ROS [Liocher and Fridovich, 2007; Imlay, 2008], condition termed as Oxidative stress. Oxidative stress affects cellular integrity only when antioxidants are no longer capable of coping with ROS [Lukaszewicz-Hussain and Moniuszko-Jakoniuk, 2004].
Hydrogen peroxide, one of the ROS is a harmful byproduct of many normal metabolic processes; to prevent damage to cells and tissues, it must be quickly converted into other, less dangerous substances. Mainly Catalase and Glutathione Peroxidase play a significant role in the elimination of hydrogen peroxide. Catalase is frequently used by cells to rapidly catalyze the decomposition of hydrogen peroxide into less-reactive gaseous oxygen and water molecules[Gaetani et. al., 1996; Yoshpe-purer and Henis, 1976]. Glutathione Peroxidase (GSHPx), a selenoenzyme that catalyses the reduction of hydrogen peroxide to water, with the simultaneous conversion of reduced glutathione to oxidised glutathione [Michiels et. al., 1994]. Glutathione, a tripeptide consisting of glutamic acid - cysteine – glycine, is the substrate for glutathione peroxidase (GSHPx), which protects cytosolic organelles from the damaging effects of the hydroperoxides formed by normal aerobic metabolism.
The present work is designed to analyse the organ wise and species wise changes in superoxide dismutase activity in a group of fish since fishes are often at the top of the aquatic chain and is one of the most appropriate organisms to study the physiological influence of changes in aquatic system because they can serve as bioindicators of environmental pollution [Dautremepuit et. al., 2004].
A lot of field studies based on the influence of various chemical substances on the Glutathione peroxidase activity in sanguine, hepatic, renal, branchial, neural and cardiac [Ramazan, et. al., 2006; Metwally and Fouad, 2008; Rajamanickam and Muthuswamy., 2009; Kandemir et. al., 2010; Nogueira et. al.., 2010; da Silva et. al., 2011; Rekha and Joseph, 2011] reported a wide spectrum of inter-site differences (higher, equal or lower activities of various antioxidant enzymes with tissue peculiarities and disbalance) in polluted compared to clean areas.
The present study is an attempt to analyse the results of species- wise and organ- wise changes in glutathione peroxidase activity in liver, gills, kidney and muscles of Oreochromis mossambicus, Mugil cephalus and Scatophagus argus
The fish were collected from a traditional aquaculture farm at Chellanam, Kochi, Kerala, India using traditional cast net. Ten fish samples of each species (O. mossambicus, M.cephalus, S.argus) of both sexes coming under similar size group (O. mossambicus with Tolal length 16.75±0.95 cm and Body weight 58.97± 12.7 g; M. cephalus with Tolal length 17.91±1.39 cm and Body weight 66.53± 12.7 g; S. argus with Tolal length 10.03±0.65 cm and Body weight 29.47± 2.42 g) were selected from the catch. The collected fishes were transported to the laboratory in living condition by keeping in polyethylene bags. On reaching the laboratory the fishes were immediately dissected and the organs Viz., kidney, liver, gills and muscle were taken, washed in ice-cold Alsever’s ringer solution, kept in plastic containers with screw cap lid and refrigerated in freezing condition. The refrigerated tissues were taken out, dried using blotting paper and the organs were weighed for the preparation of 5% of the tissue homogenate in ice-cold Tris-Hcl buffer pH 7.5 in a glass homogeniser. The prepared homogenate were centrifuged at 3500 rpm for 10 minutes in a cooling centrifuge kept at 4°C. The supernatant was collected after centrifugation and were kept in ice until the enzyme assay.
Estimation of GPx activity was carried out according to the procedure suggested by Rotruck [1973]. All the reagents used were SRL. To a reacting mixture of 0.4 M phosphate buffer pH 7.0, 10mM Sodium azide, 4mM reduced glutathione, 2.5 mM hydrogen peroxide added 200 μL of 5% homogenate solution of the sample tissue to initiate H2O2 utilization . Then the reaction of enzyme is arrested by the addition of 10% TCA at various time intervals (0 seconds, 30 seconds, 60 seconds, and 90 seconds). The test tubes were centrifuged at 3500 rpm for 10 minutes and to the supernatant0.3 M phosphate solution and 0.04% DTNB in 1% sodium citrate were added. Optical Density (OD) of colour developed was measured using a UV-Visible spectrum of spectrophotometer at 412 nm.
At last the results were statistically interpreted by the Anova test, the unifactorial pattern using SPSS version 20.
Oxidative stress, the natural consequence of the oxygen metabolism, is normally controlled by antioxidant endogenous defense systems. When these prove to be insufficient, cellular lesions develop that result in ageing but also in some pathological processes [Joanny Menvielle-Bourg, 2005]. Among the antioxidants Glutathione peroxidase play a pivotal role to alleviate the harmful effect of Hydrogen peroxide, a harmful ROS produced as a part of normal metabolic processes.
The specific activity of Glutathione peroxidase in different organs like liver, gills, muscle and kidney of Tilapia (Oreochromis mossambicus), Grey mullet (Mugil cephalus) and Spotted scat(Scatophagus argus) takes the form of graph (Figures 1,2,3 & 4).
In Tilapia (Oreochromis mossambicus) Glutathione peroxidase activity in liver, gills, kidney and muscles are 0.75 ±0.06, 0.52±0.03, and 0.34±0.02 and 0.28±0.01 μg of GSH consumed/min./mg protein respectively. Branchial Glutathione peroxidase activity is 69.33 % of hepatic; renal is 45.33 % of hepatic and 65.38% of branchial; muscular is 37.33 % of hepatic, 53.85 % of branchial and 82.35 % of Glutathione peroxidase activity.
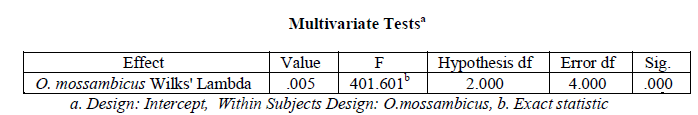
A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the organ wise variation in Superoxide dismutase activity in Oreochromis mossambicus. There was a significant variation in hepatic, branchial, renal and muscular Superoxide dismutase activity in O. mossambicus (variation in Superoxide dismutase activity with organ type), Wilks’ Lambda = 0.005, F (2, 4) = 401.601, p < .001
In Grey mullet (Mugil cephalus) the organ-wise trend is similar to but relatively higher values than in Tilapia 3.81±0.23, 3.17±0.21, 1.56±0.09, 0.99±0.06 μg of GSH consumed/min./mg protein respectively. Branchial Glutathione peroxidase is 83.2 % of hepatic, renal is 40.94 % of hepatic and 49.21% of branchial, muscular is 25.98 % of hepatic, 31.23 % of branchial and 63.46 % of renal Glutathione peroxidase activity.
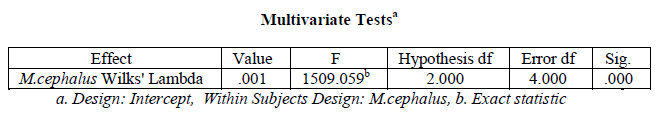
A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the organ wise variation in Superoxide dismutase activity in Mugil cephalus. There was a significant variation in hepatic, branchial, renal and muscular Superoxide dismutase in M. cephalus (variation in Superoxide dismutase activity with organ type), Wilks’ Lambda = 0.001, F (2, 4) = 1509.059, p < .001
In Spotted scat (Scatophagus argus) also the organ-wise trend is similar to Tilapia and Grey mullet, 2.3±0.09 μg of GSH consumed/min./mg protein in liver, 1.7±0.09 in gills, 0.91±0.006 in kidney and 0.66±0.007 in muscle. The branchial glutathione peroxidase is 73.91 % of hepatic, renal is 39.57% of hepatic, 53.53 % of branchial, muscular is 28.7 % of hepatic, 38.82 % of branchial and 72.53 % of renal Glutathione peroxidase activity.
A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the organ wise variation in Glutathione peroxidase activity in Scatophagus argus. There was a significant variation in hepatic, branchial, renal and muscular Glutathione peroxidase activity in S. argus (variation in Glutathione peroxidase activity with organ type), Wilks’ Lambda = 0.003, F (1, 5) = 1584.556, p < .001.
A comparison of hepatic Glutathione peroxidase activity in Oreochromis mossambicus, Mugil cephalus, Scatophagus argus shows highest activity in M.cephalus (3.81±0.23), and lesser activity in S. argus (2.3±0.09), which is 60.37 % of M.cephalus and the least in O.mossambicus (0.75±0.05) which is 19.69 % of M.cephalus and 32.61 % of S.argus hepatic Glutathione peroxidase activity.

A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the species wise variation in hepatic Glutathione peroxidase activity in three species of fish selected. There was a significant variation in hepatic Glutathione peroxidase activity in O. mossambicus, M. cephalus, S. argus (variation in hepatic Glutathione peroxidase activity with type of species), Wilks’ Lambda = 0.0004, F (1,5) = 12515.577, p < .001

A comparison of branchial glutathione peroxidase activity in Oreochromis mossambicus, Mugil cephalus, Scatophagus argus shows similar trend of hepatic Glutathione peroxidase i.e., highest activity in M. cephalus (3.17±0.21), least activity in O. mossambicus (0.52±0.03), and activity in S. argus (1.7±0.09) lies in between, which is 53.63 % of M. cephalus. Enzyme activity in O. mossambicus is16.4 % of M. cephalus and 3.59 % of S. argus. A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the species wise variation in branchial Glutathione peroxidase activity in three species of fish selected. There was a significant variation in branchial Glutathione peroxidase activity in O. mossambicus, M. cephalus, S. argus (variation in branchial Glutathione peroxidase activity with type of species), Wilks’ Lambda = 0.005, F (2,4) = 441.236, p < .001

A comparison of renal Glutathione peroxidase activity in Oreochromis mossambicus, Mugil cephalus, Scatophagus argus shows a similar trend to that of Glutathione peroxidase, i.e., highest activity in M. cephalus (1.56±0.09), and lesser activity in S. argus (0.91±0.006), which is 58.33 % of M. cephalus and the least in O. mossambicus (0.34±0.02) which is 20.79 % of M. cephalus and 37.36 % of S. argus renal Glutathione peroxidase activity.
A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the species wise variation in renal Glutathione peroxidase activity in three species of fish selected. There was a significant variation in renal Glutathione peroxidase activity in O. mossambicus, M. cephalus, S. argus (variation in renal Glutathione peroxidase activity with type of species), Wilks’ Lambda = 0.000003, F (2,4) = 753482.456, p < .001

A comparison of muscular Glutathione peroxidase activity in Oreochromis mossambicus, Mugil cephalus, Scatophagus argus shows a similar trend to that of hepatic, branchial and renal GPx activity i.e., highest activity in M.cephalus (0.99±0.06), and lesser activity in S. argus (0.66±0.007), which is 66.67 % of M. cephalus and the least in O. mossambicus (0.28±0.01) which is 28.28 % of M. cephalus and 42.42 % of S. argus muscular Glutathione peroxidase activity.
A one-way within subjects (or repeated measures) ANOVA was conducted (using SPSS version 20) to compare the species wise variation in muscular Superoxide dismutase activity in three species of fish selected. There was a significant variation in muscular Glutathione peroxidase activity in O. mossambicus, M. cephalus, S. argus (variation in muscular Glutathione peroxidase activity with type of species), Wilks’ Lambda = 0.0002, F (1,5) = 245592.429, p < .001

From the result it became clear that the selected antioxidant enzyme showed decreasing trend in the enzyme activity from Liver to muscle (Liver > Gills > Kidney > muscle). The present findings of highest hepatic Superoxide dismutase activity agree with the observations of Rajamanickam and Muthuswamy [2009] where the glutathione peroxidase activity in liver was recorded to be higher than in kidney of Common carp. Kandemir [2010] noted a decreasing trend in glutathione peroxidase activity like Liver > gills > muscle in of C.carpio L. Nogueira et. al. [2010] reported GPx activity was found to be higher in liver than in gills of armored catfish (Pterygoplichthys anisitsi) but in the case of Nile tilapia GPx showed slightly increased activity in Gills than in liver, an observation found contradictory to the present paper).
Literature search haven’t came across with similar type of study in these selected fishes especially Scatophagus argus for defending the present result of species-wise changes.
The present analysis reached at a conclusion that the Glutathione peroxidase activity show a species-wise and organwise variation with a decreasing trend like liver > gills > kidney > muscle and the species-wise variation in hepatic, branchial, renal and muscular Gutathione peroxidase activity showed similar trend like M. cephalus > S. argus > O. mossambicus.
This research work is funded by University Grant Commission, New Delhi.