- (2002) Volume 3, Issue 2
Marja-Leena Kylänpää-Bäck1, Esko Kemppainen1, Pauli Puolakkainen1,2
1Department of Surgery, Helsinki University Central Hospital. Helsinki, Finland
2The Hope Heart Institute and the University of Washington. Seattle, WA, USA
Received: 12 October 2001 Accepted: 10 January 2002
Acute pancreatitis is a common disease varying widely in severity. At present, there is no “gold standard” for the diagnosis of acute pancreatitis. Currently, the diagnosis of acute pancreatitis is based on measurements of serum amylase and/or lipase activity, which are considered unsatisfactory due to their low level of accuracy. Early identification of acute pancreatitis and especially detection of patients with a severe form of the disease is of utmost importance. Premature intrapancreatic activation of trypsinogen is a crucial early event in the athophysiology of acute pancreatitis. The conversion of trypsinogen to active trypsin is mediated by the release of its activation peptide (TAP). The active trypsin is then able to activate other pancreatic zymogens (i.e. procarboxypeptidase) leading to tissue damage and eventually to autodigestion of the pancreas. To improve the laboratory diagnostics of AP, new methods have been developed to measure this primary pancreatic proteolytic insult. Here we review the current knowledge and clinical implications of trypsin based laboratory methods and carboxypeptidase activation peptide (CAPAP) in the diagnosis and severity assessment of acute pancreatitis.
Acute Disease; Carboxypeptidases; Pancreatitis; Peptides; Trypsinogen
AP: acute pancreatitis; AUC: area under the curve; CAPAP: carboxypeptidase activation peptide; CE-CT: contrast enhanced computed tomography; CRP: C-reactive protein; ERCP: endoscopic retrograde cholangiopancreatography; MODS: multiple organ dysfunction syndrome; NPV: negative predictive value; PPV: positive predictive value; PSTI, pancreatic secretory trypsin inhibitor; ROC: receiver operating characteristics; SIRS: systemic inflammatory response syndrome; TAP: trypsinogen activation peptide; T-2 dipstick: trypsinogen-2 dipstick
Acute pancreatitis (AP) is a common emergency presentation. The incidence rate of AP varies considerably, however, in different countries. A low incidence has been reported in England (10/100,000) [1, 2] and in Germany (15/100,000) [3]. In contrast, in the USA (40-80/100,000) and in Finland (70/100,000) the incidence is high [4, 5]. AP has many distinct etiologies, though approximately 80% of all cases can be attributed to either gallstones or alcohol [6]. Also, the frequency of AP of different etiologies varies markedly in different countries [5, 7, 8, 9].
The severity of AP forms a continuum. Most of the cases are mild and conservative treatment results in rapid recovery. However, severe AP constitutes 15–20% of all cases [9, 10]. In severe AP the inflammatory process of the pancreas is often violent with frequent involvement of regional tissues and remote organ systems [11, 12, 13]. In recent decades, the mortality rate from severe AP has decreased from 30-80% to 15-20% [14]. Severe AP is now recognized to be a twophase systemic disease. In the first phase, extensive pancreatic inflammation and/or necrosis are followed by a systemic inflammatory response syndrome (SIRS) that may lead to multiple organ dysfunction syndrome (MODS) within the first week. About 50% of deaths occur during the first week of the attack, mostly from MODS [15, 16, 17, 18, 19, 20, 21]. Unless the first phase is arrested and reversed by natural defenses or therapeutic intervention, the second phase usually ensues after the second week of onset and includes the development of infected pancreatic necrosis or fluid collection with possible progression to overt sepsis, MODS and death [22, 23, 24, 25].
Organ failure is present in half of the patients with pancreatic necrosis, but the extent of pancreatic necrosis does not influence the development of remote organ complications [26, 27]. With an increasing number of failing organ systems involved in AP, the associated mortality rises [28]. The mortality associated with MODS varies between 30 and 100% [20, 26, 29, 30, 31].
The major function of pancreatic acinar cells is the synthesis, storing and secretion of powerful digestive enzymes and their inactive proenzymes, zymogens (trypsinogen, chymotrypsinogen, proelastase, procarboxypeptidases A and B and prophospholipase A2) [32, 33]. These zymogens are synthesized in the endoplasmic reticulum and then packaged into secretory granules. Following acinar cell stimulation, the content of these granules is discharged by exocytosis into the acinar lumen and passes via the pancreatic ductal system into the duodenum [34, 35]. One of the precursors, serine protease precursor trypsinogen, is the main protease in human pancreatic fluid. The conversion of trypsinogen to active trypsin, a 24-kDa protease, is normally catalyzd in the duodenum by intestinal enterokinase [34, 35, 36]. Trypsinogen is activated by proteolytic cleavage of a peptide called trypsinogen activation peptide (TAP) [37, 38]. Trypsin is the key enzyme for the rapid activation of all the proenzymes, including its own proenzyme, trypsinogen [39]. There are two major isoenzymes of trypsinogen: cationic trypsinogen-1 and anionic trypsinogen-2 [40, 41]. In healthy subjects, the ratio of trypsinogen-1 to trypsinogen-2 in pancreatic fluid is nearly fourfold and trypsinogen-1 and trypsin-1-alpha-1-antitrypsin are the major forms in serum [42].
Owing to their potent proteolytic and lipolytic functions, the secretory enzymes represent a considerable degradative (autodigestive) capacity. Compartmental intracellular transport and synthesis of secretory enzymes as inactive zymogens represent protective mechanisms against this degradation [43, 44]. The pancreatic acinar cells also synthesize the protease pancreatic secretory trypsin inhibitor (PSTI) which is considered to be the first line of defense. PSTI, a 56-amino acid polypeptide, can immediately neutralize potentially harmful trypsin intracellularly, thereby, maintaining a stable state [43]. It has been shown that even if no active trypsin was found in unstimulated pancreatic acini, there can be comparable amounts of active intracellular proteases [45]. This may be due to spontaneous intracellular protease activation. It may, however, also indicate that intracellular trypsin is immediately neutralized by local protease inhibitors.
In the normal state, only a minor proportion of the total trypsinogen production leaks into the circulation [46]. When active trypsin reaches the circulation, the major trypsin inhibitors (alpha-1-antitrypsin and alpha-2- macroglobulin) inactivate it [43]. Trypsinalpha- 1-antitrypsin complexes transfer the enzyme to alpha-2-macroglobulin before its elimination [47]. In human subjects, free alpha-2-macroglobulin has a half-life of over 100 hours [48]. Instead, the trypsin-alpha-2- macroglobulin complex is cleared from the circulation by the reticular endothelial system within 10 minutes or less [49, 50].
The pathogenesis of AP is only partially known. The initial phase involves triggering events, which are, for the most part, extrapancreatic in origin. Clinically, the most important of these are either passage of a biliary tract stone or ingestion of ethanol. Although the clinical association of AP with biliary disease and with ethanol has been firmly established, mechanistic explanations for these associations have proven elusive [51]. In experimental AP, microscopic examination of pancreatic tissue obtained after common bile-pancreatic duct ligation indicates that the earliest signs of cell injury involve acinar cells [52]. The severity of experimental AP has been directly related to the duration of duct obstruction [53].
More than 100 years ago, the premature intrapancreatic activation of trypsinogen and the subsequent activation of zymogens leading to autodigestion of the pancreas was suggested to be an essential event in the pathogenesis of AP [54]. Trypsin can be found in the normal pancreas. However, the pathological intrapancreatic activation of trypsinogen to trypsin overwhelming the inhibitory potential of PSTI leads to a more general activation of digestive enzymes in the pancreas [46, 55]. The activation of trypsinogen, in and around the pancreas, followed by the activation of other pancreatic zymogens, occurs early in the course of AP, in proportion to the extent of pancreatic injury [56, 57]. Intracellular activation of trypsinogen may be triggered by the abnormal colocalization of digestive enzyme precursors and lysosomal hydrolases [58, 59, 60]. Trypsinogen activation mediated by lysosomal hydrolase cathepsin B has been shown to be an early, as well as a critical event, leading to cell injury [61]. However, the extent of colocalization does not seem to correlate with the severity of AP and colocalisation of enzyme precursors and hydrolases has also been shown in normal acinar cells [62]. Another alternative for trypsinogen activation during secretory blockade is autoactivation, which can be considered unique for human trypsinogen [36, 63]. The disruption of the acinar cell follows premature activation of the proteases as a result of interaction between the digestive and lysosomal enzymes, and activated proteases then escape into the interstitium of the pancreas [51]. In an animal model of AP, it has been shown that there are significant quantities of uncleaved trypsinogen in the interstitial compartment suggesting that possible activation of this extracellular trypsinogen leads to autodigestion of the gland [64, 65]. Once released into the pancreatic interstitium, retroperitoneum, peritoneal cavity, and circulation, the active enzymes cause necrotising tissue damage through a variety of events, including local autodigestion by lipase and proteases eventually resulting in AP [66, 67, 68].
Currently, there is evidence that some cases of AP can be hereditary. Patients with hereditary AP have a mutation in the trypsinogen-1 gene, which makes trypsin-1 resistant to proteolytic inactivation by other intrapancreatic proteases resulting in the selfdestruction of the content of the zymogen granules [69].
Pancreatic digestive enzymes explain only part of the pathogenesis of complicated AP. The release of various inflammatory mediators is another important mechanism [24, 70]. In fact, the pathophysiology of severe AP resembles other conditions with SIRS such as sepsis, multitrauma, ischaemiareperfusion injury and burns, which do not involve the release of digestive enzymes from the pancreas [18].
Background
AP is a disease having a wide clinical variation. Patients may suffer from a multitude of symptoms, including upper abdominal pain, meteorism, abdominal resistance, fever, nausea and vomiting, ileus and jaundice [9]. None of these frequent symptoms are specific for AP or are they related to the severity of the disease. Rare clinical findings, such as ecchymosis of the flank (Grey Turner’s sign) or periumbilical area (Cullen’s sign), which occur in 1-3% of patients, also fail to effectively predict the severity of AP [71]. Within the first days of admission, patients with severe AP may develop SIRS characterized by a combination of fever, tachycardia, and tachypnea [72]. In summary, clinical findings, though helpful, are not sufficiently accurate to diagnose AP. Contrast enhanced computed tomography (CE-CT) has become the standard imaging method for diagnosing and staging AP and its complications [73, 74]. The diagnostic accuracy of CE-CT findings has proven high, with specificity approaching 100% [75]. The use of CT for a primary diagnosis is not always possible due to its limited availability and high costs [76, 77]. Furthermore, CT may be normal in 8-28 % of patients with AP, especially in mild forms of the disease [75, 78, 79, 80].
Laboratory Methods for Diagnosing AP
Amylase and Lipase
Traditionally, the biochemical diagnosis of AP is based on the determination of serum and/or urinary amylase activity [81], the activity of which increases in serum within 2- 12 hours of the onset and returns to normal within 3-5 days [82]. However, up to 19% of AP patients have a normal amylase value [83]. Furthermore, it is well-known that hyperamylasemia occurs in many extrapancreatic diseases resulting in a low specificity for AP [33]. Pancreatic lipase is synthesized, similarly to amylase, in the exocrine acinar cells and catalyzes the hydrolysis of triglycerides into diglycerides and fatty acids [33]. Wide variation in sensitivity and specificity has been reported for serum lipase determination in the diagnosis of AP, which may partially be due to different assay methods [84, 85, 86, 87, 88]. Because serum lipase remains elevated longer than serum amylase, it has been suggested that it may be useful when there is a delay between the onset of symptoms and admission [33, 82, 89, 90]. In all, however, measurement of amylase and/or lipase activity is generally considered not to be accurate enough in detecting AP.
Other Methods
There is a pressing clinical requirement for an early, simple and accurate test to improve the biochemical diagnosis of AP. Serum elastase stays elevated for up to one week after the onset of AP and may be useful in cases with delayed admission [33, 90], but the test is not routinely used. Other serum markers such as ribonuclease, chymotrypsin, phospholipase A2 and pancreatic isoamylase have been evaluated, but their use is infrequent because of limited utility [83, 90, 91, 92, 93, 94, 95].
Background
At present, the classification of AP is based on the internationally recognized Atlanta criteria [96]. According to the Atlanta classification, mild AP is associated with minimal organ dysfunction and an uneventful recovery, while AP is classified as severe if systemic and/or local complications are present. In an emergency setting, the identification of severe AP remains problematic and several patients with severe disease are diagnosed only at autopsy [97]. It has been shown that patients with severe AP and delayed transfer to intensive care unit have higher mortality than those admitted directly [98]. There is evidence that early enteral feeding, prophylactic antiobiotics and emergency endoscopic sphincterotomy in patients with biliary AP are beneficial in severe AP [8, 10, 99, 100, 101, 102, 103]. Early diagnosis of patients with severe AP, especially those with subsequent organ failure would enable their immediate referral to a centre having facilities for maximal intensive care and specialists in the endoscopic, radiological and surgical management of AP [104]. Increasing knowledge of the inflammatory process in AP has led to new therapeutic strategies aiming at modifying SIRS [24]. Moreover, since new immunomodulatory therapies may have undesirable side effects, it is of utmost importance to accurately identify patients who will benefit from immunomodulation [105, 106]. On the other hand, it is also important to recognize patients with mild course of the disease to allow them to be treated in lowercost hospital beds.
One of the main problems with AP has been the lack of accurate predictors of disease severity and the development of organ failure in the early stages of the disease. On admission, clinical assessment of severity has been shown to be unreliable [71, 107, 108]. CE-CT has improved the assessment of the disease severity by accurately identifying areas of necrosis [73, 74, 109, 110, 111]. It has been reported that necrosis of only the head of the pancreas is as dangerous as when the entire pancreas is involved [112]. Magnetic resonance imaging is being increasingly used for assessing the severity of AP with promising results [113, 114, 115]. However, organ failure in AP patients is even more problematic to predict as it occurs in only half of the patients with pancreatic necrosis [26].
There are several clinicobiochemical scoring systems for the assessment of the severity of AP [116]. The Ranson scoring system comprises 11 biochemical criteria, which require up to 48 hours for complete data collection [117]. According to a recent metaanalysis, Ranson`s signs show poor predictive power [118]. The APACHE II illness grading system is more accurate and can be used throughout the patient’s hospitalization [119, 120]. However, 12 separate measurements are needed for the APACHE II score and additional values for age and chronic health. Due to their complexity, the clinicobiochemical systems are seldom used routinely in clinical practice [121].
Laboratory Methods
Much effort has been directed to developing a single, simple, rapid, affordable and reliable laboratory test for the severity assessment of an attack of AP [63]. The severity of AP does not correlate with the level of serum amylase and lipase [83, 91, 122]. C-reactive protein (CRP) is the most commonly used laboratory test in the assessment of the severity of AP but it is useful only 48-72 hours after the onset of the disease while it is insensitive earlier [33, 38, 123]. However, in the followup during the course of the disease, CRP has proven to be useful [124]. The more recent tests, especially those for cytokines are expensive and/or laborious and timeconsuming to perform.
Immunoreactive Trypsin
There are laboratory methods based on the determination of pancreatic enzymes in serum and urine, which measure the intrinsic biological severity of organ damage by estimating the degree of the primary pancreatic proteolytic insult. Levels of immunoreactive trypsin reflect the leakage of unactivated proenzymes from injured acinar cells. The original assays for determination of immunoreactive trypsin preferentially measured cationic trypsinogen, i.e. trypsinogen-1 and abnormal concentrations were strongly considered to suggest a pancreatic course of the illness [125, 126, 127]. Recently, Appelros et al. studied immunoreactive anionic trypsinogen with an enzyme-linked immunosorbent assay, which measures anionic trypsinogen-trypsin complex with alpha-1-antitrypsin [128]. The area under the curve (AUC) of the receiver operating characteristics (ROC) curve representing the discriminatory power for severe disease was only 0.796 when immunoreactive trypsinogen was measured in urine whereas it was 0.475 in serum [128]. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 58%, 74%, 37%, 88% when measured in urine and 38%, 58%, 14%, 84% in serum. Thus, neither the measurement of immunoreactive trypsinogen in urine or in serum seems accurate enough for severity assessment of AP.
Trypsinogen-2
Using specific antibodies for trypsinogen-1 and trypsinogen-2 it was found that in AP, the serum concentrations of trypsinogen-2 were increased 50-fold and those of trypsinogen-1 only 15-fold [127]. The corresponding increase in immunoreactive trypsin was also only 15-fold [127]. Moreover, patients with AP excrete large amounts of trypsinogen-2 into urine and the concentration rises within hours of the onset of the disease. The quantitative immunofluorometric measurement for trypsinogen-2 both in urine and serum is a highly accurate marker for AP [127, 129, 130, 131]. In addition, the concentration of trypsinogen-2 shows a marked correlation with the severity of the disease (Table 1).
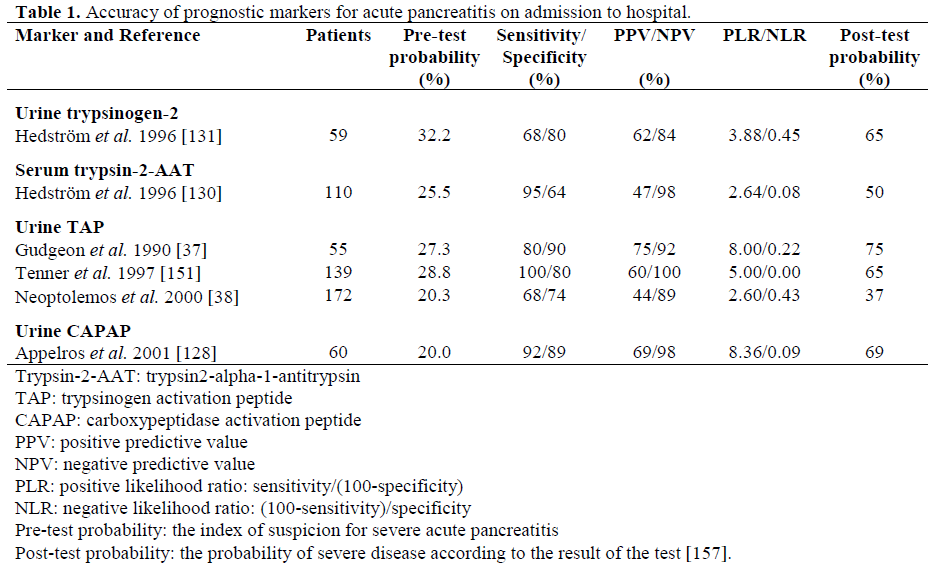
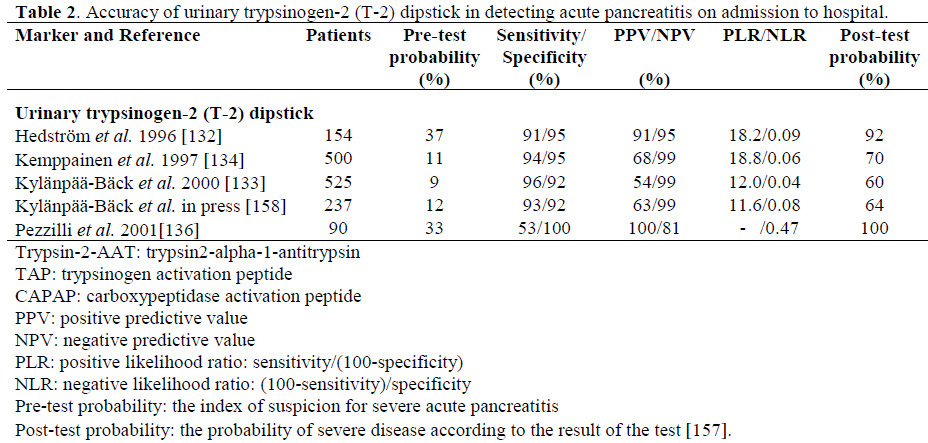
Our research group previously introduced a rapid urinary trypsinogen-2 test strip which is based on the use of immunochromatography with monoclonal antibodies [132]. The trypsinogen-2 strip test can be performed rapidly in health care centres with limited laboratory facilities. A modified 5-min trypsinogen–2 (T-2) dipstick (Medix Biochemica, Kauniainen, Finland) has recently been developed with new antibodies to detect elevated levels of trypsinogen-2 in urine. The accuracy of the T-2 dipstick test [133] proved to be very similar to that of the preliminary dipstick (sensitivity 96%, specificity 92%), which has been reported in a retrospective [132] and a prospective [134] study (Table 2). Due to the very high sensitivity (96%) and negative predictive value (NPV, 99%) of the T-2 dipstick test strip, AP can be excluded with high probability with a negative dipstick result. It, therefore, appears to be suitable as a screening test for AP in patients with acute abdominal pain. However, the positive predictive value (PPV) is relatively low (54%) indicating that the dipstick result alone cannot establish the diagnosis of AP, but additional examinations (laboratory or radiology) are needed. Earlier, trypsinogen-2 concentrations were also reported to be elevated in conditions such as hepatobiliary and pancreatic cancer, and in chronic pancreatitis [135]. The quantitative measurements of urinary trypsinogen-2 showed a good agreement with the test strip result (kappa value equal to 0.86), supporting the use of the simple and rapid dipstick [133]. In a recent report by Pezzilli et al. the T-2 dipstick test showed high specificity but low sensitivity in diagnosing AP (Table 2) [136]. The difference between those results and ours may be due to different diagnostic criteria based solely on imaging procedures and also due to possible difficulties in reading the dipstick result. In addition, urine excretion of trypsinogen could be delayed and therefore, if the time between the onset of pancreatic pain and the execution of the test is quite short (less than 6 hours), it is possible that the results of the dipstick test are negative. However, in all the studies performed so far, the dipstick has detected the severe cases of AP very accurately, which is important in clinical practice.
Trypsinogen Complexed with Antiproteases
Biochemical indicators of AP and its severity include the antiproteases and their complexes with trypsin. High serum concentrations of immunoreactive trypsin-alpha-1-antitrypsin complexes have been demonstrated in AP, and the levels on admission correlate with the severity of AP [137]. Our study group has measured trypsin-2 complexed with alpha-1- antitrypsin using a specific monoclonal antibody to trypsin-2 and a polyclonal antibody to alpha-1-antitrypsin [130]. In this study of 110 patients with AP and 66 patients with acute abdominal pain, trypsin-2-alpha-1- antitrypsin complex in serum had the largest AUC both in differentiating AP from control patients (0.995) and in detecting mild AP from the severe disease (0.82) as compared to CRP, amylase and trypsinogen-2 12 hours after admission (Table 1) [130]. Recently, the ratio of trypsin-2-alpha-1-antitrypsin to trypsinogen-1 in serum was reported to be a promising new indicator for discriminating between biliary and alcohol-induced AP [138]. Serum alpha-2-macroglobulin concentrations are found to be significantly lower in complicated attacks of AP suggesting its excessive consumption [139, 140, 141, 142, 143]. Additionally, the complexed alpha-2-macroglobulin concentrations have been shown to increase in severe AP [144]. However, measurement of neither form of alpha-2-macroglobulin is in clinical use, partially due to complicated and time-consuming assay methods.
TAP
Another potential marker for AP is TAP. Immunoreactive TAP reflects the amount of pathological intrapancreatic trypsinogen activation irrespective of whether the resulting trypsin is active or blocked by inhibitors [56, 145]. Thus, it is a marker specifically related to the onset of AP [37]. Free TAP is liberated into the peritoneal cavity and the circulation, after which, because of its small size, the peptide is rapidly cleared by the kidneys and excreted into the urine [145]. However, the measurement of TAP in urine is not a perfect test for diagnosing AP, since Gudgeon et al. reported in 1990 that 30% of patients with AP had normal TAP values on admission [37]. Further, in an endoscopic retrograde cholangiopancreatography (ERCP) study, urinary TAP was not useful in predicting mild post-ERCP AP [146]. It has also been shown that the concentrations of urinary TAP do not vary according to the cause of the disease [38].
In an animal model of AP in ascites, urine, plasma and pancreatic tissue, the TAP concentration has been shown to correlate well with the extent of pancreatic necrosis [37, 147, 148, 149, 150]. The urinary TAP/creatinine ratio correlates with the severity of the disease in humans [57]. Concentrations of TAP in urine have been shown to predict severe AP with a sensitivity of 100% and a specificity of 85% on admission to hospital within 48 hours of the onset of symptoms in an American multicenter study [151]. In a recent European multicenter study, urinary TAP showed a somewhat lower accuracy for the assessment of the severity of AP as soon as 24 hours after the onset of symptoms with a sensitivity of 58% and a specificity of 73% [38] (Table 1). However, when likelihood ratios were calculated, for example a positive urinary TAP assay 48 hours after the onset of symptoms only increased the positive likelihood ratio of severe AP from 20 to 35%. When urinary TAP measurement was combined with CRP, the probability of severe AP increased from 20 to 55% [152]. Clinically the most favourable feature of urinary TAP is the capability of differentiating between severe and mild disease during the very early phase of the disease, when other methods are not yet useful [63]. However, the general accuracy of urinary TAP alone does not qualify for clinical decision-making. In plasma, TAP showed maximal accuracy for distinction between mild and severe disease within 6 hours after admission with a sensitivity of 70% and specificity of 78%. Thereafter the prognostic accuracy declined rapidly and TAP values showed a very variable pattern possibly due to burst-like secretion [153].
Carboxypeptidase Activation Peptide (CAPAP)
In the normal state, local trypsin inhibitors inactivate trypsin in and around the pancreas. However, if trypsin activation exceeds the capacity of the trypsin inhibitors, the activation of other pancreatic zymogens occurs [56]. Therefore, released activation peptides of zymogens reflect free trypsin activity. Procarboxypeptidase B has an activation peptide, carboxypeptidase activation peptide (CAPAP), which is larger than other peptides released during proenzyme activation [154]. The large size makes it more stable and, thus, suitable for measurement in serum and urine, and now, a radioimmunoassay has been developed for this peptide [155]. It has been reported that the levels of CAPAP in urine and serum correlate well with the severity of AP [128, 156] (Table 1). The AUC, representing the discriminatory power for severe disease, was 0.9422 when CAPAP concentration was measured in urine and when measured in serum [126]. However, the study included only 60 non-consecutive AP patients and only 12 of them had severe disease. Eight of the 60 AP patients (all with mild disease) had undetectable levels of CAPAP in urine. This suggests that mild AP can occur without detectable trypsinogen activation and activation of other zymogens. It also means that measurement of CAPAP cannot be used as a diagnostic test for AP, since many mild cases would be missed. In a recent study by Pezzilli et al., though, CAPAP reached a sensitivity and specificity of 95% for diagnosing AP [156]. The study population was limited to 20 patients with AP and 20 controls. As for the accuracy of the CAPAP assay for the severity assessment of AP, further prospective clinical studies with sufficient number of patients are needed.
At present, no single biochemical marker is ideal for diagnosing and/or the early prediction of the severity of AP. Assays based on trypsin pathophysiology have brought interesting new alternatives for diagnostics and severity grading of AP. The available study results suggest the use of Actim Pancreatitis® for screening for AP in patients with acute abdominal pain. Comparison of the results in different studies is very difficult partly because there is no “gold standard” for the diagnosis of AP and also due to differences in the pre-test probability and severity grading of the disease. It has been pointed out that likelihood ratios should be used to assess the results. Urinary TAP can be measured relatively easily and has a prognostic value especially when combined with CRP and this could help physicians in clinical practice. Urinary CAPAP seems very promising as a prognostic marker but should be studied with a large consecutive series of AP patients before wider clinical use. It seems obvious, however, that no single test is ideal and accurate enough, and a combination of tests may be needed to predict severe AP and its systemic complications when new therapies are planned. In the future, additional studies with a sufficient number of patients will be needed to find out the most accurate set of markers.