Keywords
Water treatment; Precipitation; As(V); Arsenic removal; Sorption; Manganese
Introduction
Groundwater contamination with As(V) compounds is a problem for millions of people as these resources are crucial for use as drinking water [1-3]. Accumulation of arsenic compounds in the body due to use of such water poses significant health risks and may lead to arsenicosis [3]. Arsenic contamination is both natural and due to human activity. Weathering of rocks and minerals is a typical example of a natural process, while metal waste, fertilizers, fossil fuel combustion and pesticides are due to agricultural and industrial activities [4-6].
Sorption is considered as one of the most feasible water treatment techniques in arsenic removal because of its relatively low costs [7-10]. Zeolites are highly crystalline, microporous and hydrated aluminosilicate minerals, which contain alkali or alkaline cations. Clinoptilolite is a high SiO2 content heulandite group mineral and often is the main component of natural zeolites. Unmodified zeolites usually have a low affinity for arsenic due to an electrostatic repulsion between the anions and the framework of the zeolite. In such cases, surface modification, i.e. via manganese compounds, is a possible route for substantially improving oxyanion sorption [9].
Manganese compounds are used for modification of sorbents for As(V) removal from water [11,12]. Clinoptilolite modification with manganese oxides significantly enhances sorption capacity of As(V) compared to an unmodified clinoptilolite [11-13]. Mn8010CI3 -modification improves As(V) sorption capacities of zeolites [11,12]. As(V) has affinity towards crystalline Mn8010CI3 [11]. Thus, the newly observed phenomenon is important to understand and consider when Mn-modified sorbents are to be used in practice.
During sorption experiments of a mixed oxidation state Mn-modified natural zeolites (clinoptilolite) for removal of As(V) in water, an unexpected precipitation was observed, in result of which translucent greyish flakes were formed and sedimented. The aim of this short communication is to report the newly observed phenomenon, which is of significance for the removal of arsenic compounds from water using manganese-modified sorbents. This phenomenon has not been reported before to the best knowledge of the author.
Materials
Clinoptilolite-rich (clinoptilolitic content 86–94%) natural zeolite from Slovakian deposit Nižný Hrabovec was modified with a mixed oxidation state manganese compound Mn8010CI3 and was then used as a sorbent for arsenic removal. The specific weight of the unmodified material was 2200–2440 kg/m3 and porosity was 24–32%. The material also contains cristobalite, clay mica, plagioclase, rutile, quartz. Size of particles was 1–2.5 mm [12,14].
The modification method of natural zeolites with a mixed oxidation state manganese oxide used in this communication is also reported in two other works [11,12]. Conditions of the modification are also consistent with that of Mn8010CI3 synthesis described by G. Buisson [15]. 100 g of raw material were weighted and dried in air atmosphere in the oven for 1 h at 70°C. 2.5 M MnCl2 and 10 M NaOH solutions were prepared. While mixing, 100 mL of 2.5 M MnCl2 solution were added to the zeolite. Then, 1 mL of 10 M NaOH solution was added. The mixture was stirred and then aged for 24 h. After, it was placed in the oven for 3 h at 150°C, resulting in the densified modified zeolite mass. This mass was then placed in the muffle furnace and was held there for 5 h in air atmosphere at 550°C. After, it was taken out and cooled down in air at room temperature. After cooling, modified zeolite was washed with 300 mL of DI water. Material was dried in air for 1 h at room temperature, and further dried in the oven Gallenkamp Plus II (London, UK) for 4 h at 60°C. Modified material had Mn content of 8.40 ± 0.51 w%.
Unmodified natural clinoptilolite is able to sorb up to 0.36 ± 0.02 mg/g of As(V). Mn-modification increases sorbed amount of As(V) up to 13.7 times (4.92 ± 0.30 mg/g) [11]. The amount of Mn compound was the same in all the studied samples.
All compounds used during this work were of analytical grade (≥ 98%). Sodium hydroxide was obtained from Sigma- Aldrich (Riedel-de Haën, Germany). Manganese(II) chloride tetrahydrate was obtained from Firma Chempur (Piekary ?l?skie, Poland). All aqueous solutions were prepared using high purity deionized water (10 – 15 MΩ·cm), produced via water purification system Millipore Elix 3 (Billerica, USA). Arsenate solutions were prepared using disodium hydrogenarsenate heptahydrate Na2HAsO4·7H2O obtained from Alfa Aesar (Haverhill, USA).
Experimental
SEM, EDX and XRD
Scanning electron microscopy (SEM) and Energy-dispersive X-ray spectroscopy (EDX) were performed using PhenomWorld Phenom ProX (Waltham, USA) in backscattered electron regime with working voltage of 15 kV. Studied material was covered with a thin layer of gold in order to prevent charging due to electron beam. Gold sputtering was performed using Quorum Technologies Emitech K550X (Laughton, UK).
X-ray diffraction (XRD) was performed using instrument Bruker D8 Advance (Billerica, USA). Radiation source: Cu Kα, λ = 1.54180 Å; Anode voltage 40 kV; Anode current 40 mA; Kβ filter 0.02 mm thick nickel foil; slits: divergence 0.6 mm, anti-scattering 8.0 mm; scanning range: 2θ=3–55°, step 0.02°, step time 0.5 s; energy-dispersive one-dimensional detector Lynx Eye.
A combination of SEM, EDX and XRD allowed to ensure that the zeolite was successfully modified with a crystalline mixed oxidation state manganese oxide-chloride Mn8010CI3 . The details of these results are reported in another work [11].
Arsenic removal experiments
Arsenic removal or sorption experiments were conducted using a batch system. Na2HAsO4·7H2O was used for preparation of arsenic solutions of various concentrations (5, 10, 25, 50, 100, 200 and 300 mg/L). 0.5000 ± 0.0001 g sorbent was weighed in each glass vessel using analytical scales Kern ALJ 220-4 (Balingen, Germany) and 30.00 ± 0.05 mL As(V) solution was then added to every vessel. Vessels were shaken for 24 h at room temperature (23 ± 1°C) at 150 rpm using orbital shaker Biosan Multi-functional Orbital Shaker PSU-20i (Riga, Latvia) to ensure sorption equilibrium was achieved.
Results and Discussion
EDX analysis of the modified sorbent indicated that it contained 8.40 ± 0.51 w% of the Mn. EDX of the initial zeolite showed it contained no traceable amounts of Mn.
XRD diffraction patterns showed that the sorbent was modified with a new crystalline phase, which was found to be Mn8010CI3 [11,12].
SEM micrograph of the crystalline Mn8010CI3 modification compound on the surface of the zeolite is shown in Figure 1. The sorbent is also characterized and described in more detail in another works [11,12].
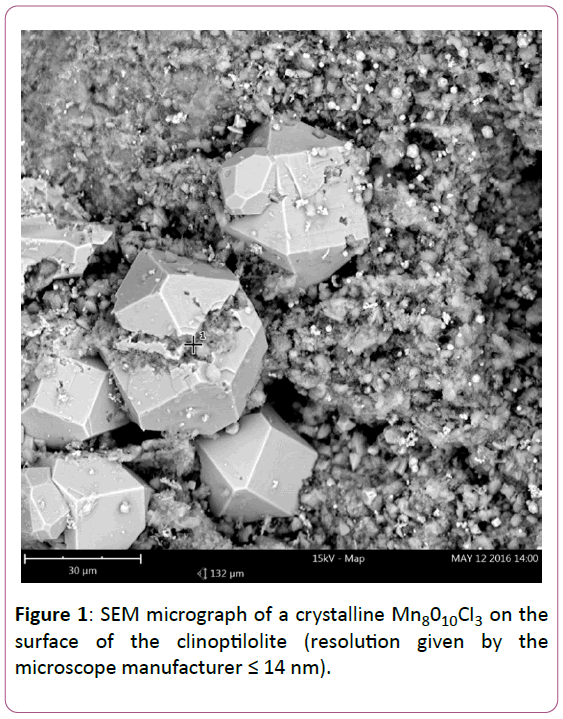
Figure 1: SEM micrograph of a crystalline Mn8010CI3 on the surface of the clinoptilolite (resolution given by the microscope manufacturer = 14 nm).
During the arsenic removal (sorption) experiments from water using a mixed oxidation state Mn-modified natural zeolites (clinoptilolite) a sudden unexpected precipitation was observed, in result of which translucent greyish flakes were formed and sedimented as shown in Figure 2.

Figure 2: Greyish precipitates formed during water treatment for As(V) removal using mixed oxidation state manganese compound Mn8010CI3 -modified clinoptilolitic zeolite.
This phenomenon has not been reported before to the best knowledge of the author. This is of significance and might have implications for the removal of arsenic compounds from water using manganese-modified sorbents. Furthermore, the quantity of formed precipitates was observed to increase with the initial concentration of As(V) present in water (from 5 to 300 mg/L, studied in this work).
Limited experimental evidence indicates that precipitates are formed by interaction of Mn8010CI3 (mixed oxidation state Mn compound) and As(V), and exist in the form of hydrates, since the precipitates are unstable in air at room temperature and moderate humidity, and degrade in air fairly quickly.
While this short communication is intended only to report a novel observation, some implications can be expected. Additional studies are required to understand the underlying mechanism and implications of such precipitation during the water treatment process with Mn-modified sorbents, raising questions on limitations of the use of such sorbents.
A possible explanation is a complexation between the zeolite-modifying compound of a mixed oxidation state Mn8010CI3 and As(V) in water with the resulting products partially precipitating. The product is likely in a form of hydrates. This mechanism is speculated by analogy with Fe-modified sorbents that are often used in arsenic removal [11,12,16,17], which form inner sphere complexes. Out of 9 mg of As(V) that were present in the 300 mg/L initial solution, only 0.18 mg could be uptaken by the unmodified zeolite, while the modified sorbent was able to adsorb 2.46 mg As(V). The difference (2.28 mg) was likely uptaken by the modification compound Mn8010CI3 itself. While speculated, this difference, possibly partially, was likely involved in the complexation.
Conclusion
In this short communication, a newly observed phenomenon was reported, being an unexpected precipitation process that occured during the As(V) sorption from an aqueous solution via Mn-modified clinoptilolite. Furthermore, with an increasing concentration of As(V) in to-be-treated water samples, the amount of formed precipitates increased proportionally. Precipitates are likely formed by interaction of Mn8010CI3 (mixed oxidation state Mn) and As(V), possibly via complexation. The precipitates had a hydrated nature and were unstable in air even at room temperature and moderate humidity, and degraded quickly outside of water solution. The concentration of As(V) in water decreased during the arsenic removal experiment indicating that part of As(V) was sorbed and was likely involved in formation of these precipitates, which was indicated by the proportionality of quantity of formed precipitates and the initial concentration of As(V) present in water solutions. While this short communication is intended only to report a novel observation, some implications can be expected. Additional studies are required to understand the underlying mechanism and implications of such precipitation during the water treatment process, raising questions on limitations of the use of such sorbents.
Acknowledgment
Andrey is thankful for an opportunity to perform this work in the laboratory of Department of Environmental Science of Latvian University. This wouldn’t be possible without prof. Maris Klavins, to whom the author is deeply grateful. Acknowledgment also goes to author’s colleagues and teachers who have supported him during this work, Juris Burlakovs, Kristine Rugele and Ruta Ozola. Andrey is especially grateful to his beloved Oksana V. Golubova.
References
- Mukherjee A, Bhattacharya P, Savage K, Foster A, Bundschuh J (2008) Distribution of geogenic arsenic in hydrologic systems: Controls and challenges. J Contam Hydrol 99: 1-7.
- Kemper K, Minnatullah K (2005) The World Bank and the Water and Sanitation Programs of South and East Asia, Technical Report No. 31303.
- Glocheux Y, Albadarin AB, Mangwandi C, Stewart E, Walker GM (2015) Production of porous aluminium and iron sulphated oxyhydroxides using industrial grade coagulants for optimised arsenic removal from groundwater. J Ind Eng Chem 25: 56-66.
- A textbook of modern toxicology, 3rd edn., ed. by Hodgson E. John Wiley and Sons, Hoboken, New Jersey (2004).
- https://www.geog.cam.ac.uk/research/projects/arsenic/symposium/S1.2_P_Ravenscroft.pdf
- Bhattacharya P, Claesson M, Bundschuh J, Sracek O, Fagerberg J, et al. (2006) Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Sci Total Environ 358: 97-120.
- Ansone-Bertina L, Klavins M (2016) Sorption of V and VI group metalloids (As, Sb, Te) on modified peat sorbents. Open Chem 14: 46-59.
- Dupont L, Jolly G, Aplincourt M (2007)Arsenic adsorption on lignocellulosic substrate loaded with ferric ion. Environ Chem Lett 5: 125-129.
- Figueiredo H, Quintelas C (2014) Tailored zeolites for the removal of metal oxyanions: Overcoming intrinsic limitations of zeolites. J Hazard Mater 274: 287-299.
- Zhang FS, Itoh H (2005) Iron oxide-loaded slag for arsenic removal from aqueous system. Chemosphere 60: 319-325.
- Krauklis AE, Ozola R, Burlakovs J, Rugele K, Kirillov K, et al. (2017) FeOOH and Mn8010CI3 Modified Zeolites for As(V) Removal in Aqueous Medium. J Chem Technol Biotechnol (Special Issue).
- Krauklis AE (2018) Use of Synthetic and Natural Zeolites Tailored for As(V) Sorption, in Zeolites and Their Applications, ed. M. Nageeb Rashed, Intech Open.
- Camacho LM, Parra RR, Deng S (2011)Arsenic removal from groundwater by MnO2-modified natural clinoptilolite zeolite: Effects of pH and initial feed concentration. J Hazard Mater 189: 286-293.
- https://www.vskpro-zeo.sk/en/zeolite/natural/Slovakian natural zeolite
- Buisson G (1976) Preparation and properties of a manganese oxychloride of formula Mn8010CI3. J Solid State Chem 19: 175-178.
- Lakshmipathiraj P, Narasimhan BRV, Prabhakar S, Bhaskar RG (2006) Adsorption of arsenate on synthetic goethite from aqueous solutions. J Hazard Mater 136: 281-287.
- Guo X, Fuhua C (2005) Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ Sci Technol 39: 6808-6818.