Keywords
Epidemiology; Intervention; Valve; Valvular; Aortic; Mitral; Tricuspid
Introduction
The valvular heart diseases (VHD) cause a great burden worldwide. For practical purposes, they are grouped as PVD if alteration arises on the valve apparatus or secondary if alteration is due to lesions in other structures (e.g, aortic root disease, myocardial disease). The degenerative, rheumatic and congenital valve diseases represent most types of VHD present in medical records and, although the numerous specific VHD described may pose a diagnostic challenge (Table 1), a good clinical evaluation and the diagnostic arsenal now available permit a proper diagnosis in the majority of cases. Those patients with severe VHD commonly progress to a clinical state that requires valvular structural intervention in order to reestablish valve function and improve prognosis. In this manner, interventional cardiology and cardiovascular surgery have experienced a revolution since the beginning of the century, with the outcome of novel technologies for transcatheter treatments, minimally invasive surgery and less invasive approaches. The benefits of transcatheter valve therapy (TVT) for VHD range from improvement of symptoms and quality of life to reduction on mortality in selected groups [1-3]. The role of the Heart Team is critical for the identification, selection and appropriate implementation of the chosen treatment. The final treatment is the product of a discussion and common understanding of the specialized caregivers involved in these therapeutic modalities [4,5]. We conducted a clinical review with focus on the new intervention modalities so far available for treatment of aortic, mitral and tricuspid valve heart diseases. Most of the following epidemiology and treatment discussion will concern the prevalent causes of VHD. Rare entities like lupus, thrombotic non-infectious endocarditis and drug induced valvular disease will not be the focus of this review.
| Primary valve diseases (PVD) |
| Congenital (e.g. bicuspid aortic valve, Fallot Tetralogy) |
| Infectious (e.g. endocarditis, abscess) |
| Genetic (e.g. Marfan syndrome, Fabry's Disease) |
| Auto-immune (e.g. Rheumatic Heart Disease, Lupus, Rheumatoid Arthritis) |
| Age-related (e.g. calcific lesions) |
| Carcinoid Syndrome |
| Drug-related (e.g. Methysergide) |
| Diet Medicines (e.g. Fenfluramine, Phentermine) |
| Radiation therapy |
| Others (e.g. catheter related trauma, non-infectious endocarditis) |
| Secondary valve diseases |
| Aortic root diseases (e.g. Marfan syndrome, Syphilis aortitis, Auto-immune arteritis) |
| Annulus dilation secondary to ventricular dilation (e.g. end stage heart failure) |
| Papillary muscle dysfunction secondary to ischemic insult |
| Others (e.g. atrial mixoma causing valve dysfunction) |
Table 1: Causes of valvular heart diseases (VHD).
Materials and Methods
We searched Pubmed by using the terms “valve”, “valvular”, "aortic”, “mitral” and “tricuspid” from 2001 to september 30, 2015, and identified studies and papers about the epidemiology and intervention modalities for the treatment of valve heart diseases. We included prospective, retrospective and observational studies that analyzed the epidemiology of valve heart diseases and the efficacy and security of new therapeutic modalities compared to traditional ones, specially the consolidated surgery interventions. We also included case records that explored the same issues. Studies included present good quality and analyses, allowing us making trustable conclusions.
Results and Discussion
The VHD incidence rises with age. Data from population-studies point to a prevalence up to 13,3% in the 75 years and older group [6]. Its true prevalence is better stated when echocardiographic screening is performed because the clinical suspicion is inaccurate to detect patients without advanced lesions [7]. An USA population-based study performed in the community of Olmsted County, MN, estimated the prevalence of moderate or severe valvular disease to be 1.8% on the basis of symptoms or cardiac murmur auscultation. This contrasts with a prevalence of 2.5% determined by echocardiographic findings, suggesting that the burden of VHD is underestimated when assessed by clinical data [6]. The Euro Heart Survey [8] assessed the etiologies of the various types of valvular disease according to echocardiographic image plus surgical findings when available. The degenerative diseases were the most common etiology, representing 63% of all cases of native heart valve disease. The rheumatic heart disease (RHD) came next, accounting for 22% of all patients [8]. This supports the understanding of a changing in the epidemiology of valvular diseases in the last 60 years, with a shift from rheumatic to degenerative disease as the main etiology of primary valve diseases (PVD) in industrialized countries. A less expressive changing is noticed in developing countries. A recent chinese epidemical study showed RHD is still the leading etiology of VHD in Southern China [9] and a recent Turkish survey on 1300 patients hospitalized in 2009 revealed that RHD accounted for 46% of all valve diseases, followed by degenerative etiologies in 29% [10] (Table 1).
Some works have shown a male preponderance among elderly with aortic stenosis, although these differences may be due to population bias [11]. Studies performed in developing countries have shown a female preponderance among patients with diagnosis of VHD and this is likely attributed to the great importance of RHD as the main cause of VHD in these areas. In a South African centre, RHD accounted for 72% of VHD in 2006- 2007, and, of the 344 patients presenting with RHD, 68% were women, with a median age of 43 years at diagnosis [12]. In the Turkish Survey, the median age were 57 years, being women responsible for 60% of the cases [5].
Surgery treatment of VHD represents more than 20% of the heart surgeries performed in industrialized countries nowadays, but the transcatheter interventions have expanded their potential to treat a broader and more heterogeneous patient population [13]. A heart team guided decision is now the mainstay for the treatment of patients with VHD [14].
The rheumatic heart diseases
From all etiologic groups of VHD, RHD represents perhaps the most easily subjected to public control, albeit still very prevalent (Figure 1) [15]. Its preventable nature and the damage control so long possible through social and medical interventions (i.e. peniciline benzatine application) were not enough to consistently reduce the burden of this condition. Even today, RHD deteriorates life`s quality and life expectancy of a great amount of children and young people, most of them located in the poor regions of the globe, where overcrowding is common and health care limited [15,16]. The global incidence of acute rheumatic fever ranges between 5 and 51 cases/100.000 individuals-year in the 5 to 15-years old group, being the highest incidence (100- 200/100.000) in Eastern Europe, Middle East and Australasia [17]. In 2010, the prevalence of RHD among Australia’s Aboriginal and Torres Strait Islander indigenous people were 6,45/1000, 26 times higher than in non-Indigenous Australians [18]. Latin America has an incidence of acute rheumatic fever of 21,000 cases/year. Epidemiological data in Brazil are scarce. Official organs estimate an rheumatic fever incidence of 3% among children and adolescents. This makes RHD responsible for 40% of the heart surgeries performed in the country [19].
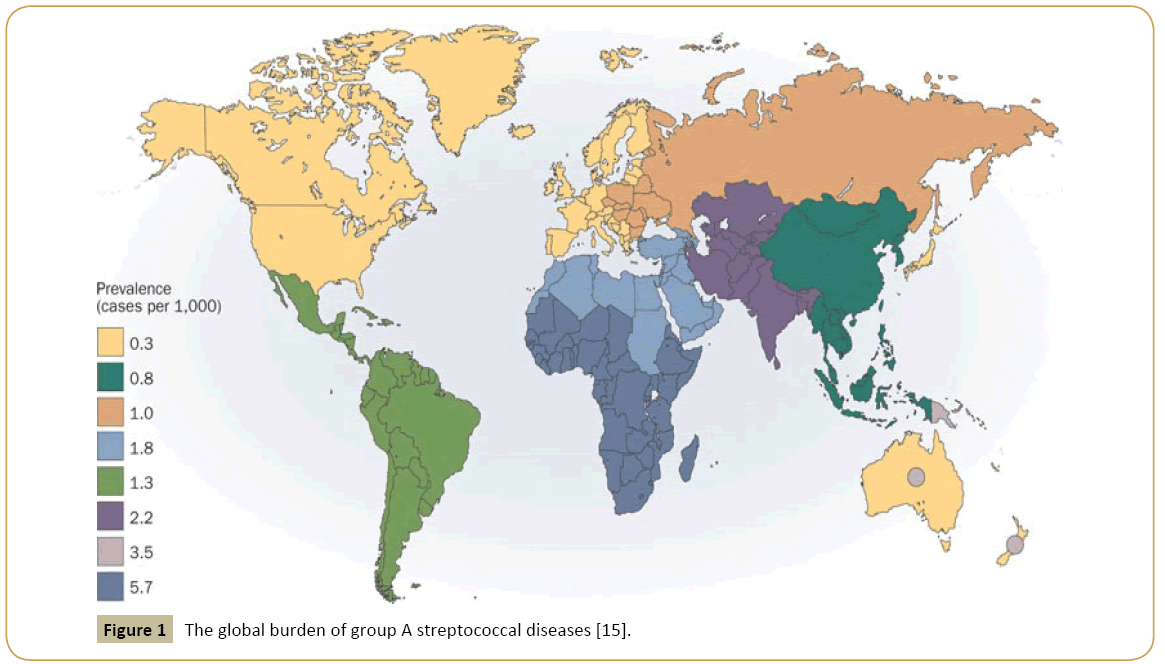
Figure 1: The global burden of group A streptococcal diseases [15].
Transcatheter therapies in valvular heart diseases
Patients with severe VHD commonly progress to a clinical state that requires valvular structural intervention to reestablish valve function and improve prognosis. In this manner, interventional cardiology and cardiovascular surgery have experienced a revolution since the beginning of the century, with the outcome of novel technologies for transcatheter treatments, minimally invasive surgery and less invasive approaches. In that increasingly old and complex patient population, the benefits of transcatheter valve therapy (TVT) for VHD range from improvement of symptoms and quality of life to reduction on mortality in selected groups [20-22]. The role of the Heart Team is critical for the identification, selection and appropriate implementation of the chosen treatment. Its success depends on the right integration of clinical, radiological, echocardiographic and surgical data, customized to patient’s expectations and preferences and respecting the principles of bioethics [23-25]. The final treatment is the product of a discussion and common understanding of the specialized caregivers involved in these therapeutic modalities [24,25].
In current practice, TVT are indicated to patients based on their short-term risk to surgery. Operative risk can be estimated by scoring systems as the Society of Thoracic Surgeons score (STS) or the European System for Cardiac Operative Risk Evaluation (EuroSCORE) [24]. There are limitations to these scores, including the fact they do not take into consideration anatomical factors, major organ system compromise, some comorbidities and the frailty of the patient. The presence of specific anatomical factors like very calcified aorta (“porcelain aorta”), which prevents aortic cross-clamping, previous thoracic radiotherapy exposure, multiple previous sternotomies and certain thoracic deformities may work as procedure impediments and turn the patients technically inoperable. In this way, the final morbidity and mortality risks must be estimated with risk-score calculation for surgery and careful individualized evaluation [23].
Aortic valve diseases
The aortic valve has a major role in cardiac fitness. There are a lot of different aortic valve lesions so far recognized and the diverse image findings it generates still intrigue [26]. In United States, the VHD accounts for 10% to 20% of all cardiac surgical procedures, being the aortic valve replacement (AVR) responsible for two thirds of all [13,27]. Aortic stenosis is responsible for most of the AVR performed. It carries a prevalence of 0,2% among individuals between 50 and 59 years, increasing to 9.8% in octogenarians [28]. The aortic regurgitation represents a smaller fraction of the patients treated by surgery or transcatheter intervention, being, in some series, most cases associated with aortic root disease [27].
Transcatheter aortic valve implantation
Surgical aortic valve replacement (SAVR) is the mainstay of treatment of symptomatic aortic stenosis (AS). In properly selected patients, this surgical procedure offers substantial improvements in symptoms and life expectancy (Figure 2). However, approximately one third of patients are not referred to surgery and the reasons include the high risk of heart surgery in elderly patients and patients with many comorbidities [29]. Elderly patients with symptomatic AS carry a higher surgical risk and must be routinely considered for transcatheter aortic valve implantation (TAVI) in qualified centers.
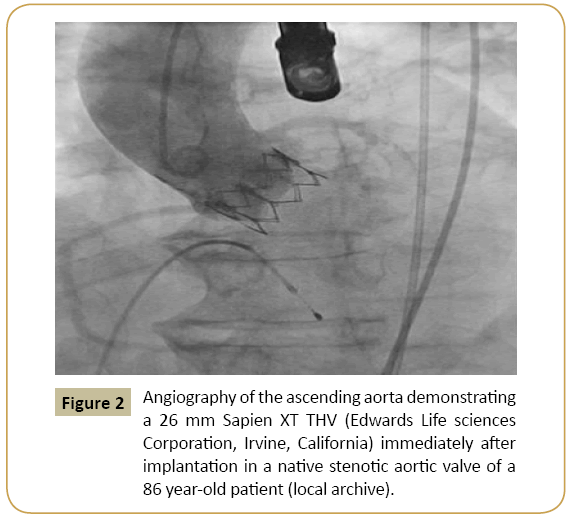
Figure 2: Angiography of the ascending aorta demonstrating a 26 mm Sapien XT THV (Edwards Life sciences Corporation, Irvine, California) immediately after implantation in a native stenotic aortic valve of a 86 year-old patient (local archive).
Since the first human TAVI in 2002 [18], it has been studied in numerous observational studies, randomized trials and multicenter registries that included large numbers of high-risk patients with severe symptomatic AS. The TAVI`s feasibility, safety and efficacy in that population have been increasingly demonstrated. Currently, eligible patients for TAVI must have severe AS, echocardiographically characterized by resting or inducible aortic peak velocity ≥ 4.0 m/s or mean pressure gradient ≥ 40 mmHg, besides a valve area <1.0 cm2 or indexed valve area <0.6 cm2/m2. Symptoms, typically effort dyspnea, angina or syncope must be present. In the patients group deemed unsuitable for surgery, TAVI brings a significant clinical improvement, with absolute reduction on all cause mortality risk of 21.8% in five years when compared to standard clinical treatment [8]. These patients are considered inoperable by an estimated probability of death or serious irreversible morbidity after SAVR of more than 50%, a prohibitive risk that is calculated by risk scores and/or by the presence of comorbidities or anatomic factors that preclude or increase the risk of cardiac surgery [22]. In patients considered to be operable but with high surgical risk for SAVR, defined by the Heart Team as a predicted risk of death of more than 15% in 30 days, TAVI is a safe alternative to surgery, demonstrating sustained hemodynamic and clinical improvements, with left ventricular mass regression and mortality rate similar to SAVR in a short and long term fashion [22,24]. In this high risk population, TAVI presents a higher risk of major vascular complications and stroke at 30 days, ranging from 3-5%, but with similar long-term cumulative incidence [20,30]. In other hand, SAVR presents higher rates of atrial fibrillation, major bleeding and acute kidney injury [22,31].
In regard to valve function, the only important difference between SAVR and TAVI is the rate of paravalvular regurgitation (PVR). The first generation transcatheter heart valves (THV) presented higher rates of PVR compared to surgically implanted valves due to many factors, including eccentric shape or severely calcified aortic annulus. They lead to undersizing of the THV, malapposition or displacement of the prosthesis into a high or low position within the aortic root [32]. Even the presence of mild aortic regurgitation may be associated with worse prognosis [20]. However, the occurrence of moderate or severe PVR (>2+) has the most significant impact on prognosis after TAVI, with a two- to four-time increase in 1-year mortality risk compared to patients without clinically significant PVR [33]. In such scenario, balloon post-dilatation of the valve or implantation of a second valve (valve-in-valve) are possible interventions to correct the leakage. The achievement of lower rates of PVR may, thus, further increase survival after TAVI. A recently published randomized controlled trial compared TAVI with self-expandable THV and SAVR in high-risk surgical patients and demonstrated lower rates of moderate or severe PVR than previous trials, besides a 2 year survival advantage for TAVI patients [22]. The routine use of three dimensional imaging techniques, mainly multidetector computed tomography (MDCT) and 3D transesophageal echocardiogram, improve annulus sizing, resulting in a better selection of properly sized valves [22,34]. Newer generation transcatheter valves with better aortic root sealing properties and also increased worldwide procedure operators experience may lead to better long term outcomes in terms of paravalvular leaks and vascular complications. Central transvalvular regurgitation is a rare event and occurs on a similar rate between the two modalities of treatment, at short and long term [20].
The access route for implantation of the prosthesis should be thoroughly discussed by the Heart Team. Images from thoracic and abdominal aorta, iliac, femoral and subclavian arteries, obtained by multidetector computed tomography (MDCT), magnetic resonance or invasive angiogram are routinely used for evaluation of tortuosity, obstructive atherosclerotic disease, calcifications, measurement of the vascular diameters and thus selection of the most suitable access route. In contemporary daily practice, the transfemoral access route is the preferred way in numerous TAVI centres and has been used in most procedures [35,36]. Transfemoral TAVI is usually performed as a true percutaneous procedure through a needle puncture of the common femoral artery, subsequent arterial dilatation for sheath and TAVI delivery system insertion, and a final vascular closure with suture-based devices to ensure hemostasis. However, inadequate choice of the femoral route can result in severe vascular and hemorrhagic complications. In these cases, it is preferable to use alternative access routes as the subclavian, transaortic or transapical route, with the active participation of heart surgeons. Although the risk of vascular complications was significant in early studies, this risk has been continuously decreasing due to the reduction on sheaths diameters used in procedures, being initially 22F to 24F2 (3) and ranging from 14F to 18F with current generation prothesis [33].
Among the most frequent complications encountered after TAVI are conduction disturbances and subsequent requirement for permanent pacemaker implantation (PPI). Most large scale studies or registries report a PPI rate of 6.8 to 38% [7,9,20,37]. The anatomical proximity of the aortic valve to the conduction system accounts for the speculated mechanism of conduction tissue injury, which is believed to be a mechanical compression by the prosthesis, particularly with longer stent frames, selfexpanding prostheses, pre- or postdilatation and deep implant depth. The use of a self-expandable valve has been identified as an independent predictor for the need of PPI when compared to a balloon-expandable device [33]. Also, the presence of right bundle branch block (RBBB) at baseline was significantly associated with increased need for PPI, as opposed to pre-existing left bundle branch block (LBBB) [38]. After the procedure, a temporary pacemaker should be left in place for 24-48h. Careful monitoring until discharge is recommended, since AV block may occur within a few days after the procedure. Furthermore, excessive oversizing and deep implant positioning should be avoided. Chronotropic medication should be adjusted to a minimum or discontinued. PPI is not associated with any increase in mortality after 30 days or long term follow-up of 2 years. Indeed, it shows a protective effect concerning the occurrence of unexpected death [39].
In high risk patients, TAVI is well established as an effective and safe treatment for degenerative native aortic valves with severe stenosis [20,23] (Figures 3 and 4). However, in this highly heterogenous group, there are subgroups that deserve special attention given their impact on TAVI results and cost-benefit ratios. In different studies, the subgroup of patients with chronic lung disease (CLD) had a higher mortality after one year of treatment compared to non-CLD patients [34,40]. A lack of TAVI benefit in these patients may be predicted by a pre-procedure shorter distance walked at the 6-min walk test [41]. Although CLD patients undergoing TAVI have a worse outcomes than patients without CLD, TAVI continue to be a better approach than conservative therapy for this subgroup [22]. Transfemoral TAVI using only sedation and avoiding endotracheal intubation should be the preferred strategy in this scenario.
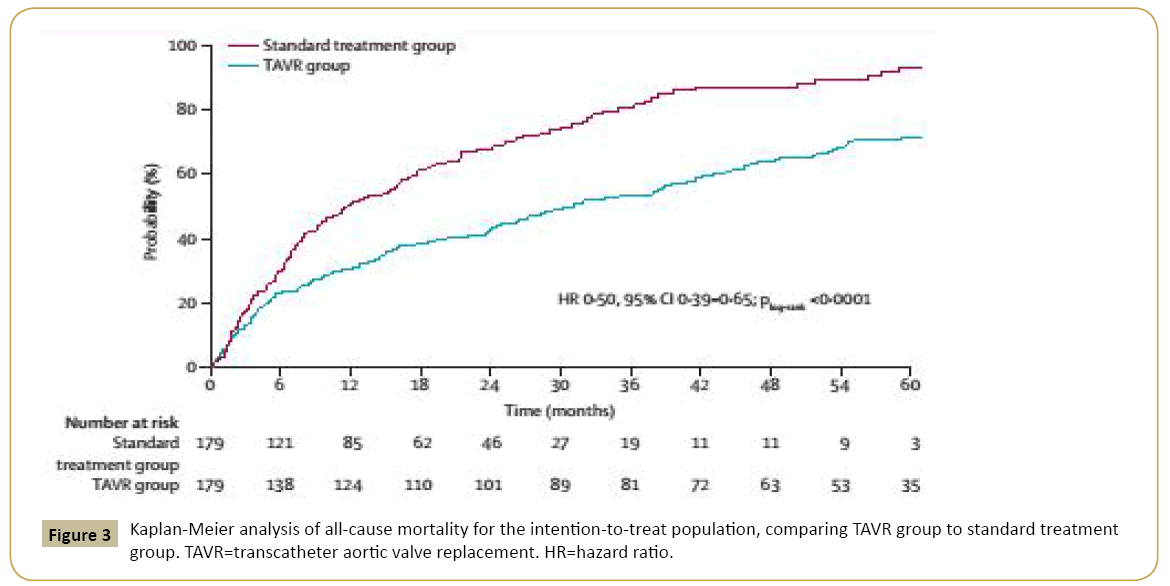
Figure 3: Kaplan-Meier analysis of all-cause mortality for the intention-to-treat population, comparing TAVR group to standard treatment group. TAVR=transcatheter aortic valve replacement. HR=hazard ratio.
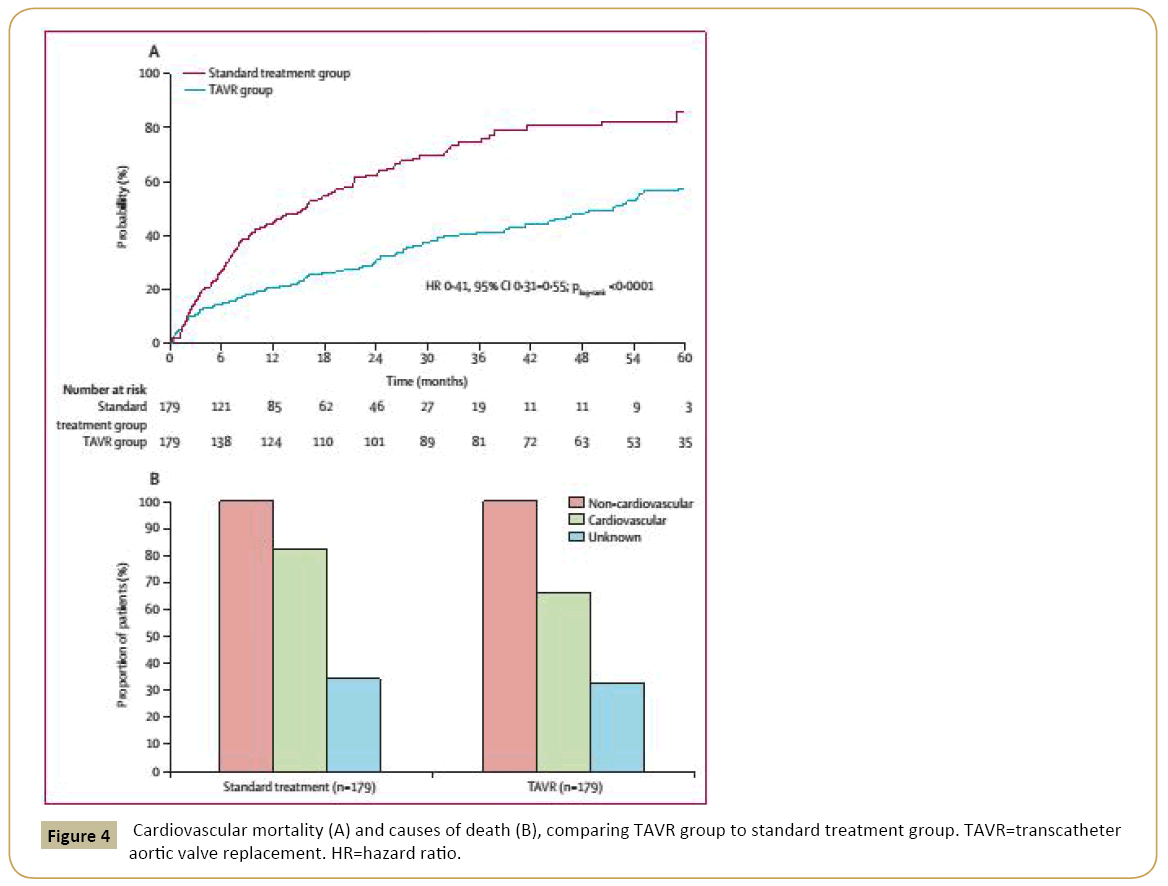
Figure 4: Cardiovascular mortality (A) and causes of death (B), comparing TAVR group to standard treatment group. TAVR=transcatheter aortic valve replacement. HR=hazard ratio.
Coronary artery disease (CAD) is another condition frequently diagnosed in patients with AS. AS is associated with a 50% increased risk of cardiovascular death and myocardial infarction over approximately 5 years of follow-up [27]. The standard treatment choice for patients with AS and CAD has been surgical aortic valve replacement (SAVR) and concomitant coronary artery bypass grafting (CABG). This practice has shown to reduce the rates of perioperative MI and mortality when compared to patients not undergoing simultaneous CABG, even though the combined operation carries a small but real increased risk of mortality [24]. However, in the setting of less-invasive transcatheter therapy, the appropriate management of coexistent significant CAD still remains unclear since those patients were systematically excluded from the largest randomized trials. Meanwhile, registries and small-scale studies have provided a considerable amount of data in regard to the safety and feasibility of percutaneous coronary intervention (PCI) before, at the same time or after TAVI [42]. Currently, ongoing large randomized trials with hard endpoints intend to address some of the aspects and outcomes of this population group. Until their results are published, an individualized approach of the cases by the Heart Team should guide selection of the patients for PCI. Coronary angiography is strongly recommended in the assessment of the extent of the coronary disease, the feasibility of PCI if revascularization is being considered and the eligibility for TAVI. In our practice, we have had favorable results in patients with severe AS and CAD submitted to TAVI with either prior PCI or conservative coronary treatment, using Heart Team individualized based decisions.
Device improvements and an increasing number of studies involving different valve patients groups point to possible benefits and applicability of this technology in a wider range of scenarios. Bicuspid aortic valve anatomy and dysfunctional aortic valve with severe aortic regurgitation were once considered absolute contraindications for TAVI, mainly due to the occurrence of postimplantation PVR, being carriers of those conditions excluded from the largest randomized trials. However, small-scale studies have shown that PVR rates may be mitigated by proper THV sizing with MDCT [43], and, despite the increased risk of usage of a second valve (valve-in-valve, as described above), the one year mortality rates have been acceptable [44]. Therefore, TAVI may be an effective alternative for the treatment of severe symptomatic aortic regurgitation or AS in patients with a congenitally bicuspid valve, in properly selected high-risk patients.
Mitral valve diseases
The mitral valve disease has a different epidemiology from aortic valve disease, being RHD the main cause of primary mitral stenosis and regurgitation. Even though, mitral regurgitation secondary to heart failure with dilated chamber has earned a high position in the ranking, being a common finding in patients with terminal heart failure [34]. In the RHD group, surgery and transcutaneous intervention have a clear role and should be a heart team guided treatment [45]. On the other hand, the direct valve intervention of secondary mitral disease has raised frequent debate about its efficacy and security [32,35].
Transcatheter mitral valve interventions
Percutaneous transcatheter intervention on mitral valve is a wellestablished treatment for mitral stenosis. Several randomized controlled trials have demonstrated the safety and efficacy of percutaneous balloon mitral commissurotomy (PBMC) compared to surgical commissurotomy. Patients with symptomatic mitral stenosis are referred for PBMC if they have favorable valve morphology in the absence of left atrial thrombus or in the case of high-risk patients for surgery with a moderate-to-severe mitral regurgitation demonstrated on echocardiogram. Mitral valve surgery is recommended for patients with unfavorable echocardiographic findings for PBMC or for whom previous PMBC have failed [23].
In the setting of mitral regurgitation (MR), valve surgery is the default treatment for severe or symptomatic disease. Surgical mitral valve repair yields superior outcomes when compared to valve replacement in patients with degenerative (or primary) disease, whereas in patients with functional (or secondary) MR due to ischemic heart disease or dilated cardiomyopathy, the benefits of repair over replacement are less clear. The mitral valve`s structural complexity and unique anatomic location combined to its diverse pathologic alterations have motivated the development of numerous surgical valve repair and replacement techniques over the past decades. Some of these concepts have also been used in the development of transcatheter mitral valve treatment. In the last decade, a growing number of studies have evaluated novel devices and transcatheter techniques as for repair of mitral valve leaflets, annulus and chordae tendineae as for replacement of the diseased valve with a prosthesis implant. To date, the greatest clinical experience is with leaflet repair by an edge-to-edge coaptation, in which the anterior and posterior leaflets are brought together to create a double valve orifice and reduce regurgitation. This approach is based on the surgical technique described by Alfieri et al [46]. Selected patients who have been treated with this surgical technique as a stand-alone procedure (without annuloplasty) have had successful results lasting up to 12 years, providing a background to validate the transcatheter technique. By far, the greatest transcatheter experience has been with the MitraClip device (Abbott Vascular, Santa Clara, California), a 4-mm-wide cobalt-chromium implant with two arms that are opened and closed with the use of the delivery-system handle [47]. The procedure is performed under general anesthesia, with the use of fluoroscopic and transesophageal echocardiographic guidance. A venous needle puncture is performed, followed by right heart catheterization and atrial transeptal puncture. The device is steered until it is aligned over the origin of the regurgitant jet, being advanced into the left ventricle. The mitral leaflets are grasped and the device is closed in order to approximate the leaflets. Selection criteria are not wide and the eligible patients must fulfill anatomical criteria to submit to clip implantation. The regurgitant jet must be centered at the level of the coaptation zone, in the central two-thirds of the coaptation line. There must be a coaptation length of at least 2 mm and a depth below the mitral annular plane of no more than 11 mm for coaptation between the leaflets. The need for some coaptation length excludes patients with an extremely dilated mitral annulus, which causes the leaflet edges to be pulled apart. In this anatomic setting, annuloplasty is likely necessary. One of the leaflets must be flail and the gap and the width of the flail segment cannot be more than 10 and 15 mm, respectively. There must not be severe leaflet or annular calcification. The baseline mitral valve area should be greater than 4 cm2 because placement of the clip significantly diminishes the mitral valve area. Careful attention to these details on preprocedure echocardiographic evaluation is necessary to ensure a successful device implantation and avoid complications.
The MitraClip™ approach has been compared to standard surgical repair and replacement in a large randomized trial [48]. Patients with non-rheumatic grade 3+ or 4+ chronic MR were included. For inclusion, the patients had to be either symptomatic with a left ventricular ejection fraction (LVEF) of more than 25% or asymptomatic having pulmonary hypertension or ventricular dysfunction (LVEF of no less than 25%). In four years, these low to moderate surgical risk patients submitted to MitraClip™ device had similar mortality compared to the surgical approach, with higher rates of residual MR requiring surgical procedures and smaller improvement in left ventricular dimensions.
Most of the surgeries required because of procedure failure were observed during the first year of follow-up, with rates of 20.4% in the percutaneous-repair group and 2.2% in the surgery group. In the percutaneous-repair group, surgery was needed predominantly due to no implantation of a device or to MR of grade 3+ or 4+ after attachment of a device to a single leaflet. After 12 months, the rate of patients free from grades 3+ or 4+ MR remained stable, being the proportion of new mitral valve interventions very low and similar between the two groups. Functional improvement measured by a reduction in the NYHA class was similar between the percutaneous and surgical groups at 1 year and 4 years after procedures. In other hand, the MitraClip™ device had a higher safety profile when compared to surgical therapy, with a lower risk of blood transfusions during the first 30 days [48]. The difference in effectiveness between the transcatheter and the surgical treatments was mainly observed in the group of patients with degenerative MR. In the subset of patients with functional MR, surgery was less effective in comparison to the subset of patients with degenerative MR, and the results were comparable to transcatheter treatment. Patients with more than 70 years of age also presented similar efficacy results between the two treatments.
The MitraClip™ device was also evaluated in the treatment of patients with 3 to 4+ grade MR and a high surgical risk, defined as a predicted 30-day mortality of 12% or more based on the STS risk calculator [48]. In this high-risk population, a 30-day mortality of 4.8% was observed with no deaths related to device failure. At 12 months, MR was ≤ 2+ in 84% of patients, with significant improvements in symptoms and left ventricle dimensions. Therefore, transcatheter mitral valve repair is an effective and safe alternative option to surgery in high-risk patients, with chronic 3 to 4+ grade MR and a favorable anatomy of the mitral apparatus. Appropriate pre-, intra-, and postprocedure evaluation of MR patients is critical and possibly the most complex evaluation of the various valve lesions amenable to any form of transcatheter therapy. The success of treatment will heavily depend on a multidisciplinary approach that includes the echo cardiographer, clinical cardiologist, cardiac surgeon and interventional cardiologist.
Tricuspid valve diseases
The diagnosis of tricuspid valve disease is not unusual in clinical practice. The finding of a mild-to-moderate tricuspid regurgitation is common in echocardiographic evaluation and is generally well tolerated. Most cases of tricuspid regurgitation are secondary to right ventricular enlargement and tricuspid annular dilation [36]. Tricuspid stenosis is a rare condition in developed countries and almost always has a rheumatic origin [49].
Transcatheter therapies for tricuspid and pulmonary valve disease
Severe TR is associated to right heart failure, worse prognosis and does not predictably improve after treatment of the left-sided valve lesion. Thereby, tricuspid valve surgery is recommended for patients with secondary TR undergoing left-sided valve surgery, as well as for patients with primary TR with refractory symptoms. Tricuspid valve repair, largely focused on annuloplasty, is preferred over replacement [23].
Currently, transcatheter interventions for tricuspid valve disease have been restricted primarily to patients with a degenerating bioprosthesis. Percutaneous therapies for direct native tricuspid valve intervention are still lacking, due in part to the diverse annular dimensions routinely found in severe TR and to some anatomic issues shared with the mitral valve, like the absence of a rigid landing zone for valve deployment. Recently, the Mitralign® Percutaneous Annuloplasty System (MPAS), originally designed to remodel the mitral annulus, has been successfully implanted in an 89 year-old woman with severe isolated TR, deemed high risk for open heart surgery [50]. The procedure was performed by a trans-jugular venous approach under general anesthesia and was echo cardiographically guided. A significant reduction in annular area and effective regurgitant orifice area resulted in a marked reduction in TR, improvements in right atrial pressure and left ventricle stroke volume. Future studies properly designed are needed to assess the efficacy and safety of that and other transcatheter therapeutic techniques for tricuspid valve.
In regard to the pulmonary valve, transcatheter percutaneous interventions are well stablished and include balloon valvuloplasty for pulmonary valve stenosis and percutaneous bio prosthetic valve implantation for treating right ventricular outflow tract/ pulmonary trunk dysfunction. These diseases will not be addressed in this review.
Prosthetic valves
TVT may also be applied in the setting of failed surgically implanted valve prosthesis. In this group of patients, reoperation to replace a dysfunctional prosthetic heart valve is a serious medical event. Mortality related to a second valve surgery due to prosthetic valve leaflet dysfunction or periprosthetic leak is approximately 10%, being higher than 15% if concomitant coronary artery bypass grafting is also required [51,52]. In regard to prosthesis types, mortality may range from 8.6% for replacement of a tissue valve to 26% for replacement of a mechanical valve [51].
In degenerated bioprosthetic valves, transcatheter approach became possible using valve-in-valve implantation, in which the new THV is inserted inside the degenerated surgically implanted bioprosthesis. Valve-in-valve implantation has been successfully performed in degenerated aortic, mitral, pulmonic and tricuspid bio prostheses, as well as in pulmonary conduits. The presence of a previously implanted bioprosthesis provides an ideal landing platform for THVs, no matter if there is stenosis or regurgitation of the prosthetic valve, being generally easier in patients with stented bio prostheses than in stent less ones. Procedural success in aortic position was reported to be as high as 93%, with 1-year survival of 83% and improved outcomes with primary prosthetic regurgitation in the VIVID (Valve-in-Valve International Data) registry, the largest experience so far published [50]. There were no differences between the THVs used, the self-expandable Core Valve (Medtronic) and the balloon-expandable Edwards SAPIEN devices (Edwards Lifesciences) in terms of mortality or stroke rates. The incidence of patient-prosthesis mismatch was lower in patients with regurgitation as the predominant mechanism of failure at baseline (19% vs. 36%; p < 0.001). Data from the VIVID registry will soon elucidate the efficacy and safety of this intervention on the mitral position, where the majority of THVs implantations are performed, using a surgical trans apical approach. Assessment of the mechanism of bioprosthetic failure, determination of the inner diameter of the prosthetic valve, determination of the perfect size of the THV and choosing the right access site are necessary prerequisites before performing the procedure. Transesophageal echocardiogram and MDCT are routinely used on this assessment.
Patients with failed surgical aortic valves secondary to stenosis should be separated into those with degenerated valves, to whom valve-in-valve implantation is reasonable, and those who have elevated gradients and small effective orifice area as a result of severe patient-prosthesis mismatch. In the later group, a valve-in-valve procedure may not reduce the pressure gradient across the valve or may even worsen it. In patients with bioprosthetic valve regurgitation, clinicians must clarify whether it is transvalvular regurgitation, from mechanical leaflet malfunction or structural degeneration, or a paravalvular regurgitation. Although the former may be successfully treated using valve-in-valve therapy, the latter is not suitable for such techniques. Transthoracic or transesophageal echocardiogram are generally appropriate for that differentiation. Patients with prosthetic paravalvular regurgitation who are deemed unsuitable for reoperation may benefit from other percutaneous techniques to occlude the paravalvular leak (PVL) that may be occluded by the off-label use of several devices originally designed for treatment of cardiac congenital defects (atrial or ventricular septal defect occluders) or for vascular embolization or occlusion (Amplatzer Vascular Plugs, St. Jude Medical, St. Paul, Minnesota). Aortic bioprosthetic or mechanical PVL may be percutaneously approached by a retrograde technique and most of them require a single device for closure, although more can be placed, if necessary. Mitral bioprosthetic and mechanical PVL can be approached by an antegrade cannulation technique through right heart catheterization and transeptal puncture. However, access to the mitral valve can be challenge, especially for posteriorly and medially located PVL, as a result of unfavorable angulation. In this cases, the transapical access can offer a more precise and accurate approach to the mitral PVL and ultimately can lead to a decrease in procedural time.
Potential complications may occur due to device overhanging, which may lead to obstruction of the coronary ostia, valvular flow derangement in the setting of a narrow left ventricle outflow tract and prosthetic dysfunction, particularly with mechanical prostheses. Imaging assessment of leak reduction is performed during the procedure and, once the operator is satisfied with both reduction in degree of leak to mild (or less, if possible) and normal leaflet function (particularly for a mechanical valve), the devices are released. Three dimensional imaging modalities, including 3D TEE and computed tomography with 3D/4D reconstruction, are important for preprocedural planning and intraprocedural guidance.
Infective endocarditis is a common cause of prosthetic valves failure and also a strong contraindication for transcatheter therapy, because the necessary debridement of infected tissue is not possible with this modality of treatment. Thus, even the slightest suspicion of an acute or subacute endocarditis needs to be excluded before implantation of a THV or occluder devices.
Cost-effectiveness of transcatheter heart valves
In the high-risk elderly group of patients, the morbidity and level of symptoms may be greater concerns than mortality. A marked and durable improvement in functional class and quality of life after TAVI or Percutaneous Mitral Valve Repair has been well demonstrated [21,22]. Due to the increasingly higher medical experience and technological improvements, TAVI might be currently performed in experienced centers as a purely percutaneous procedure in a conscious patient under local anaesthesia. This results in reduction of procedure time and overall hospital length of stay.
The randomized PARTNER trials documented a marked reduction in rehospitalization with transfemoral TAVI as compared with medical management and, in comparison to surgery, a significantly shorter length of stay as well as an earlier improvement in functional status [20,34]. Evaluating the direct incremental on cost-effectiveness of TAVI compared to medical therapy in the PARTNER B trial, transcatheter valve treatment was associated with higher medical care costs during the initial hospitalization and lower costs during the first following year because of reduced rehospitalization rate [53]. Comparing the direct costeffectiveness of TAVI with SAVR in the PARTNER A trial, similar one-year costs and quality-adjusted life years were observed. However, a stratified subanalysis according to access route demonstrated that TAVI performed through transapical access resulted in higher costs and less quality-adjusted life expectancy compared with SAVR. In other hand, transfemoral TAVI appeared to be attractive from an economical point of view, with lower costs for one year after TAVI and higher health-adjusted life expectancy when compared to transapical TAVI and SAVR [53]. These results, however, represent the first TAVI experience of most of the participating centers and may have been influenced by the learning curve, especially with the transapical approach [21].
Conclusion
THVs are high cost devices conceived for implantation in inoperable patients or in patients deemed as high-risk for surgical complications. In this complex population, transcatheter valve replacement is justified by its potential clinical benefits, including shorter stays in the intensive care unit and hospital, earlier improvement in functional status, reduction in rehospitalization rate and improvement in life expectancy. All these advantages may have a substantial impact on health-economic outcomes. In order to achieve such favorable cost-effectiveness ratio, it is appropriate to defer any type of intervention in patients who will not benefit from it in terms of symptoms or life span improvement, even with a successful procedure. For them, such intervention would be considered futile [23]. Additionally, TVI in lower risk patients is still a matter of debate, mainly due to the possible shift in cost-effectiveness ratios in a long term basis compared to conventional surgical treatments. In this population, ongoing studies of TAVI will help to clarify this and other issues in near future.
Acknowledgment
We thank the Hospital Ana Nery and the patients we care everyday for the continuous motivation and scientific stimulus.
References
- Mack M, Leon M, Smith C, Miller D, Moses J, et al. (2015) 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis: a randomised controlled trial. The Lancet 385: 2477-2484.
- Kapadia S, Leon M, Makkar R, Tuzcu E, Svensson L, et al. (2015) 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis: a randomised controlled trial. The Lancet 385: 2485-2491.
- Holmes D, Rich J, Zoghbi W, Mack M (2013) The Heart Team of Cardiovascular Care. Journal of the American College of Cardiology 61: 903-907.
- Lancellotti P, Rosenhek R, Pibarot P, Iung B, Otto C, et al. (2013) ESC Working Group on Valvular Heart Disease Position Paper--heart valve clinics: organization, structure, and experiences. European Heart Journal 34: 1597-1606.
- Rosenhek R, Iung B, Tornos P, Antunes M, Prendergast B, et al. (2011) ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. European Heart Journal 33: 822-828.
- Nkomo V, Gardin J, Skelton T, Gottdiener J, Scott C, et al. (2006). Burden of valvular heart diseases: a population-based study. The Lancet 368: 1005-1011.
- Iung B, Vahanian A (2014) Epidemiology of Acquired Valvular Heart Disease. Canadian Journal of Cardiology 30: 962-970.
- Iung B, Baron G, Tornos P, Gohlke-BÃrwolf C, Butchart E, et al. (2007) Valvular Heart Disease in the Community: A European Experience. Current Problems in Cardiology 32: 609-661.
- Fangzhou L, Shulin W, Yumei X, Wei W, Xianzhang Z, et al. (2014) GW25-e3549 Epidemiological survey of valvular heart disease in five years: Changes in prevalence, etiological spectrum and management in a single cardiovascular center of Southern China. Journal of the American College of Cardiology 64: C97.
- The Turkish registry of heart valve disease (2013) Arch Turk Soc Cardiol 41: 1-10.
- d'Arcy J, Prendergast B, Chambers J, Ray S, Bridgewater B (2010) Valvular heart disease: the next cardiac epidemic. Heart 97: 91-93.
- Sliwa K, Carrington M, Mayosi B, Zigiriadis E, Mvungi R, et al. (2009) Incidence and characteristics of newly diagnosed rheumatic heart disease in Urban African adults: insights from the Heart of Soweto Study. European Heart Journal 31: 719-727.
- Iung B, Vahanian A (2011) Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 8: 162-172.
- Zhang P (2014) AHA/ACC Guideline for the Patients with Valvular Heart Disease. Cell Biochem Biophys 72: 829-831.
- Carapetis J, Steer A, Mulholland E, Weber M (2005) The global burden of group A streptococcal diseases. The Lancet Infectious Diseases5: 685-694.
- Essop M, Peters F (2014) Contemporary Issues in Rheumatic Fever and Chronic Rheumatic Heart Disease. Circulation 130: 2181-2188.
- Pastore S, De Cunto A, Benettoni A, Berton E, Taddio A, et al. (2010) The resurgence of rheumatic fever in a developed country area: the role of echocardiography. Rheumatology 50: 396-400.
- Russell E, Maguire G (2015) APSC2015-1125 A Review of Outcome Following Valve Surgery for Rheumatic Heart Disease in Australia. Global Heart 10: e2-e3.
- Seckeler M, Hoke T (2011) The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clinical Epidemiology 67.
- 22. Mack M, Leon M, Smith C, Miller D, Moses J, et al. (2015) 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis: a randomised controlled trial. The Lancet 385: 2477-2484.
- Kapadia S, Leon M, Makkar R, Tuzcu E, Svensson L, et al. (2015) 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis: a randomised controlled trial. The Lancet 385: 2485-2491.
- Holmes D, Rich J, Zoghbi W, Mack M (2013) The Heart Team of Cardiovascular Care. Journal of the American College of Cardiology 61: 903-907.
- Nishimura R, Otto C, Bonow R, Carabello B, Erwin J, et al. (2014) AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129: 2440-2492.
- Lancellotti P, Rosenhek R, Pibarot P, Iung B, Otto C, et al. (2013) ESC Working Group on Valvular Heart Disease Position Paper--heart valve clinics: organization, structure, and experiences. European Heart Journal 34: 1597-1606.
- Rosenhek R, Iung B, Tornos P, Antunes M, Prendergast B, et al. (2011) ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. European Heart Journal 33: 822-828.
- Manka R, Binter C, Kozerke S (2014) Hurricane aorta. The Lancet 384: 2141.
- Otto C, Bonow R (2014) Valvular heart disease. Philadelphia, PA: Elsevier/Saunders; 2014.
- Otto C, Prendergast B (2014) Aortic-Valve Stenosis - From Patients at Risk to Severe Valve Obstruction. New England Journal of Medicine 371: 744-756.
- Charlson E, Legedza A, Hamel M (2006) Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis 15: 312-321.
- Smith CR, Leon M, Mack MJ (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364: 2187-2198.
- Delgado V, Ng A, van de Veire N, van der Kley F, Schuijf J, et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. European Heart Journal 31: 1114-1123.
- Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G et al. (2012) Cross-Sectional Computed Tomographic Assessment Improves Accuracy of Aortic Annular Sizing for Transcatheter Aortic Valve Replacement and Reduces the Incidence of Paravalvular Aortic Regurgitation. Journal of the American College of Cardiology 59: 1275-1286.
- Abdel-Wahab M, Neumann F, Mehilli J, Frerker C, Richardt D, et al. (2015) 1-Year Outcomes After Transcatheter Aortic Valve Replacement With Balloon-Expandable Versus Self-Expandable Valves. Journal of the American College of Cardiology 66: 791-800.
- deBrito F, Carvalho L, Sarmento-Leite R, Mangione J, Lemos P, et al. (2015) Outcomes and predictors of mortality after transcatheter aortic valve implantation: Results of the Brazilian registry. Cathet Cardiovasc Intervent 85: E153-E162.
- Holmes D, Brennan J, Rumsfeld J, Dai D, O'Brien S, et al. (2015) Clinical Outcomes at 1 Year Following Transcatheter Aortic Valve Replacement. JAMA 313: 1019.
- Nijhoff F, Abawi M, Agostoni P, Ramjankhan F, Doevendans P, et al. (2015) 'Transcatheter Aortic Valve Implantation With the New Balloon-Expandable Sapien 3 Versus Sapien XT Valve System: A Propensity Score-Matched Single-Center Compar-ison'. Circulation: Cardiovascular Interventions 8: e002408-e002408.
- Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, et al. (2015) Surgical Treatment of Moderate Ischemic Mitral Regurgitation. New England Journal of Medicine 372: 1770-1774.
- Nazif T, Dizon J, Hahn R, Xu K, Babaliaros V, et al. (2015) Predictors and Clinical Outcomes of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JACC: Cardiovascular Interventions 8: 60-69.
- Dvir D, Waksman R, Barbash I, Svensson L, Tuzcu E, et al. (2012) TCT-94 Outcomes Of Patients With Severe Aortic Stenosis And Chronic Obstructive Pulmonary Disease Treated With Transcatheter Versus Surgical Aortic Valve Replacement Versus Medical Therapy. Journal of the American College of Cardiology 60: B29-B30.
- Paradis J, Fried J, Nazif T, Kirtane A, Harjai K, et al. (2014) Aortic stenosis and coronary artery disease: What do we know? What don't we know? A comprehensive review of the literature with proposed treatment algorithms. European Heart Journal 35: 2069-2082.
- Mylotte D, Lefevre T, Watanabe Y, Sondergaard L, Windecker S, et al. (2014) Transcatheter aortic valve replacement in bicuspid aortic valve disease. Journal of the American College of Cardiology 63: A1939.
- Roy D, Schaefer U, Guetta V, Hildick-Smith D, Mollmann H, et al. (2013) Transcatheter Aortic Valve Implantation for Pure Severe Native Aortic Valve Regurgitation. Journal of the American College of Cardiology. 61: 1577-1584.
- Alfieri O, Maisano F, De Bonis M, Stefano P, Torracca L, et al. (2001) The double-orifice technique in mitral valve repair: A simple solution for complex problems. The Journal of Thoracic and Cardiovascular Surgery. 122: 674-681.
- Timek T (2003) Edge-to-Edge Mitral Valve Repair Without Ring Annuloplasty for Acute Ischemic Mitral Regurgitation. Circulation 108: 122-127.
- Lung B (2003) A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European Heart Journal 24: 1231-1243.
- Maisano F, Vigano V, Blasio A, Colombo A, Calabrese C, et al. (2006) Surgical isolated edge-to-edge mitral valve repair without annuloplasty: clinical proof of the principle for an endovascular approach. EuroIntervention 2: 181-186, 2006.
- Jones J, O'Kane H, Gladstone D, Sarsam M, Campalani G, et al. (2001) Repeat heart valve surgery: Risk factors for operative mortality. The Journal of Thoracic and Cardiovascular Surgery 122: 913-918.
- Eggebrecht H, Schelle S, Puls M, Plicht B, von Bardeleben R, et al. (2015) Risk and outcomes of complications during and after MitraClip implantation: Experience in 828 patients from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Cathet Cardiovasc Intervent 86: 728-735.
- Bruce C, Connolly H (2009) Right-Sided Valve Disease Deserves a Little More Respect. Circulation 119: 2726-2734.
- Schofer J, Bijuklic K, Tiburtius C, Hansen L, Groothuis A, et al. (2015) First-in-Human Transcatheter Tricuspid Valve Repair in a Patient With Severely Regurgitant Tricuspid Valve. Journal of the American College of Cardiology 65: 1190-1195.
- Dvir D, Webb J (2015) Transcatheter Aortic Valve-in-Valve Implantation for Patients With Degenerative Surgical Bioprosthetic Valves. Circulation Journal 79: 695-703.
- Gossl M, Rihal C (2013) Percutaneous Treatment of Aortic and Mitral Valve Paravalvular Regurgitation. Current Cardiology Reports 15.
- Reynolds M, Magnuson E, Wang K, Lei Y, Vilain K, et al. (2012) Cost-Effectiveness of Transcatheter Aortic Valve Replacement Compared With Standard Care Among Inoperable Patients With Severe Aortic Stenosis: Results From the Placement of Aortic Transcatheter Valves (PARTNER) Trial (Cohort B). Circulation 125: 1102-1109.