Keywords
Vitamin D; Vitamin D analog; Cathelicidin LL-37; Antimicrobial peptide; Wound healing; Proliferation
Introduction
A variety of pathways for the “perioprotective” function of vitamin D have been elucidated. An important property of vitamin D is its ability to induce the antimicrobial peptide, human cathelicidin LL-37 [1-3], and increase expression of the genes coding for microbial pattern recognition receptors [4]. LL-37 has the ability to eliminate microbes through diverse mechanisms, which might play a key role in restricting the development of resistant bacterial strains [5]. In addition to its anti-microbial activities, LL-37 has been demonstrated to elicit a plethora of pleiotropic effects. LL-37 has the ability to strengthen the innate barrier, reduce pro-inflammatory cytokines, and stimulate proliferation and migration of epithelial cells [5,6]. LL-37 has also been reported to inhibit in vitro osteoclastogenesis and down-regulate expression of osteoclast genes [7]. Monocytes, neutrophils and subsets of T lymphocytes have chemotactic receptors that respond to LL-37 [8]. LL-37 induces proliferation and migration of human epithelial cells and enhances activities which promote wound healing [8-10]. The topical use of vitamin D in psoriasis (chronic inflammatory skin disease) has been shown to increase LL-37 levels and suppress the production of pro-inflammatory cytokines IL-12/23, IL-1α, IL-1β, and TNF-α [11].
A potential role of vitamin D in periodontal health is supported by many different findings. Variant polymorphisms of the vitamin D receptor gene are associated with an increased risk for periodontal disease and tooth loss [1,12]. Patients with sufficient serum baseline values of vitamin D had significantly better clinical attachment gains and pocket depth reductions following periodontal surgery when compared to patients who had lower baseline values of vitamin D [13]. McMahon et al. using the active form of vitamin D, found that increasing its concentration was directly related to an increase in the amount of human cathelicidin LL-37 produced by gingival epithelial cells. Incubation of these cells in the presence of vitamin D with the periodontal pathogen, Aggregatibacter actinomycetemcomitans, led to a significant increase in antibacterial activity [14].
In longitudinal studies, patients taking supplemental doses of vitamin D were found to have better periodontal clinical parameters than those not taking vitamin D supplements [15-17]. These patients had been taking supplements for an average of 10 years, and they had statistically significantly improved probing depths, less attachment loss and less bone loss than those patients not taking supplementation. All this emerging evidence suggests that vitamin D has an important role in influencing the immune response, wound repair, and antimicrobial activity, which may prove beneficial in the management of chronic inflammatory diseases, such as periodontitis. Vitamin D may prove efficacious as an adjunct to traditional periodontal therapy due to its ability to induce LL-37 production in periodontal tissue.
Preliminary evidence for the effect of vitamin D deficiency on periodontal surgery outcomes demonstrated less clinical attachment gain after surgery compared with control patients [13]. Other studies showed that vitamin D and vitamin D analogs were actually anti-proliferative agents and may be useful in anti-cancer therapy [18-20]. These differences may be due to the local environment, the concentration of vitamin D being used, and the effects on multiple cellular functions.
Vitamin D, despite its well-demonstrated beneficial properties, can cause hypercalcemia [21], but there are analogs, including 20hydroxyD3 (20D3), which are equally as potent as vitamin D but do not cause hypercalcemia [22,23]. The purpose of this study was to determine potential benefits of vitamin D and the non-calcemic 20D3 vitamin D analog on oral wound healing, via production of LL-37. The amount of LL-37 produced by a gingival epithelial cell line was measured following in vitro stimulation with vitamin D or 20D3, and the effects of both on proliferation and in vitro wound healing, via LL-37, were evaluated. The hypothesis tested was that vitamin D and 20D3 regulate gingival epithelial cell function through the induction of LL-37 which may facilitate oral wound healing.
Materials and Methods
Human gingival epithelial cell line
Human gingival epithelial cells (S-G) [24] were obtained from Dr. Kasten's FH, East Tennessee State University, Quillen College of Medicine, Johnson City, TN [25-27]. These cells have been used in several published studies [28-32]. The cells were grown in Dulbecco's Modified Eagle Medium (DMEM; Gibco; Grand Island NY), supplemented with 10% (v/v) newborn calf serum (NCS) (Gibco) and 100 μg/ml gentamicin (Sigma-Aldrich; St. Louis, MO) (termed growth medium) at 37°C in a humidified atmosphere of 5% CO2 in air.
Vitamin D, vitamin D analog, and anti-LL-37
1,25(OH)2D3 and the analog, 20(OH)D3, were kindly provided by Dr. Slominski (University of Tennessee Health Science Center, Memphis TN). Vitamin D or the analog (5 μg) were solubilized in absolute ethanol to make 0.1 mM stock solutions, and then stored at -80°C. Neutralizing anti-human LL-37 (mouse monoclonal antibody, IgG1, low endotoxin) was obtained from Hycult Biotech Inc., (Plymouth Meeting, PA), and an isotype control mouse monoclonal antibody was obtained from R&D Systems (Minneapolis, MN).
Effect of vitamin D and vitamin D analog on cell viability
Effects of vitamin D and vitamin D analog on S-G epithelial cell viability were assessed by determining their effects on the ability of the cells to cleave the tetrazolium salt (3-[4, 5-dimethylthiazol- 2-yl]-2,5-diphenyl tetrazolium bromide) (MTT) to a formazan dye, using a kit from Boehringer Mannheim Corp. (Indianapolis, IN). These solutions were made by diluting stock vitamin D or analog solutions in DMEM containing 100 μg/ml gentamicin (DMEMgent) so that the final ethanol concentration was 1%. To obtain conditioned media, the cells were seeded at 2.5 × 104 cells/well in 96-well plates, in growth medium and cultured for 48 hours at 37°C to achieve complete attachment and spreading of the cells. The growth medium was then removed, and the wells were washed once with PBS and then DMEM-gent with or without vitamin D or the vitamin D analog (0.1 nM-1 μM; final ethanol concentration: 1%) was added. Control medium was DMEM-gent containing 1% ethanol. After an exposure period of 6 days, MTT was added to the cells at a final concentration of 0.5 mg/ml and incubated for 4 hr at 37°C. Purple formazan crystals produced from the MTT by metabolically active cells were solubilized by overnight exposure to a solubilization solution provided in the kit, at 37°C. Absorbance was read at 540 nm using a microtiter plate spectrophotometer (SPECTROstarNano; BMG Labtech GmbH; Offenburg, Germany). Results were expressed as % control (A540nm in cells exposed to DMEM-gent only).
LL-37 production
Production of LL-37 was measured in S-G-conditioned media using a commercially available ELISA kit (Hycult Biotech). To obtain conditioned media, the cells were seeded at 2.0 × 105 cells/well in 24 well plates, in growth medium and cultured for 48 hours at 37°C to achieve complete attachment and spreading of the cells. The growth medium was then removed, and the wells washed once with PBS and then DMEM-gent with or without vitamin D or the vitamin D analog (0.1 nM-100 nM; final ethanol concentration: 1%) was added. Control medium was DMEM-gent and 1% ethanol. After incubation for 1-6 days, the cell supernatants were harvested and assayed for LL-37, per the procedure outlined in the kit. The sensitivity of this ELISA is 0.1 ± ng/ml.
Cell proliferation
Proliferation was determined by measuring the incorporation of 5-bromo-2’-deoxyuridine (BrdU) into cellular DNA. This technique is based on the incorporation of the pyrimidine analogue BrdU instead of thymidine into the DNA of proliferating cells. After it is incorporated into DNA, BrdU is detected by immunoassay, using a colorimetric BrdU Cell Proliferation ELISA (Boehringer Mannheim Corp., Indianapolis, IN). S-G cells (2.0 × 103) were seeded into wells of 96-well plates, and cells were cultured in growth medium for 48 h at 37°C to achieve complete attachment and spreading of the cells. This medium was removed and serum-free DMEMgent was added and incubated for 24 hr to render the cells synchronous and quiescent (G0-arrested) [33]. The medium was then removed and DMEM-gent containing 0.1% NCS and vitamin D or the analog were added to the cells for 4 days (so that the final concentration of EtOH was 1%). Control medium was DMEMgent containing 0.1% NCS and 1% EtOH. BrdU was then added to the cells and incubated for 4 hr. The cells were fixed and their DNA denatured to improve the accessibility of the incorporated BrdU for detection by an anti-BrdU peroxidase-conjugated monoclonal antibody (which has no cross-reactivity with any endogenous cellular components such as thymidine, uridine, or DNA). Peroxidase substrate was added, and the colored reaction product measured by determining absorbance at 450 nm using a microtiter plate spectrophotometer. The absorbance values directly correlate to the amount of DNA synthesis and thereby to the number of proliferating cells. The data were expressed as % control (Abs. 450 nm in cells exposed to DMEM-gent containing 0.1% NCS and 1% EtOH).
Effects of neutralizing anti-LL-37 on proliferation
S-G cells (2.0 × 103) were seeded into wells of 96-well plates, and cells were cultured in growth medium for 48 h at 37°C to achieve complete attachment and spreading of the cells. This medium was removed and serum-free DMEM-gent was added and incubated for 24 h to render the cells synchronous and quiescent. The medium was then removed and DMEM-gent containing 0.1% NCS and vitamin D or the analog (1 × 10-8 M), with or without neutralizing anti-LL-37 or the isotype control antibody, were added to the cells for 4 days. Final EtOH concentration in these solutions was 1%, and control cultures were exposed to DMEM-gent containing 0.1% NCS and 1% EtOH. Some cultures were exposed to neutralizing LL-37 or control antibody only. In all cultures, final ethanol concentration was 1%. Antibodies were added in estimated 6-, 10-, or 20-fold molar excess (~1.3 x 10-9 M, 2.2 x 10-9 M, and 4.4 × 10-9 M, respectively) over LL-37 levels [estimated based on previous experiments (above)]. After this incubation period, BrdU was added and the assay was carried out as described above.
In vitro wound assay
Wound assays were performed as previously described [8,34,35] in order to assess the effects of vitamin D and the analog on the migration potential of S-G cells. Briefly, S-G cells were grown to confluency in growth medium to create monolayers in 24-well tissue culture plates. Cells were serum starved with DMEM gent containing 0.1% NCS for 4 hours after reaching confluency and then a wound was created in each well with a sterile 200 μL pipet tip. The wounded monolayers were washed twice with phosphate-buffered saline in order to remove any detached or loose cells. The cells were then treated with 10 nM of vitamin D or the analog in combination with a neutralizing antibody or the isotype control antibody at 37°C and 5% CO2 in DMEM-gent containing 0.1% NCS for 12 hours. A time lapse microscope (Zeiss AXIO Observer Z1, Zeiss 102404853, Jena, Germany) was used for the wound assays. The 24-well plates were placed on a motorized stage equipped with an environmental chamber and maintained at 37°C and 5% CO2. Time-lapse images were collected with an Axio Cam MRC camera every 30 minutes using the Zeiss AxioVision software (Zeiss). Images were compiled to generate QuickTime movies. The same fields of the wound margin were photographed and compared at the different time points, during the 12-hour viewing period, and the distance covered by the cells in the wound area was calculated for each condition using Image 1.38 J × software (NIH; Bethesda, MD). Wound assay experiments were repeated three times with duplicate samples for each condition.
Statistical analysis
All experiments were performed with triplicate samples, unless otherwise noted. Data values are expressed as mean ± standard deviation and analyzed using a one-way analysis of variance (ANOVA), and multivariate analysis variance (MNOVA), and Scheffe’s F procedure for post hoc comparisons, using StatView® software. Two blinded examiners (AN and AK) measured the distance travelled by the S-G cells in the wound assays and the intra- and inter-examiner reliability was assessed with the intraclass correlation coefficient (ICC) using the IBM® SPSS® statistics software (Version 22). The intraexaminer reliability for the assessment of the distance travelled by the S-G cells at 6 and 12 hours was 0.940 [95% confidence interval (CI) 0.731-0.988] for blinded examiner AN. The interexaminer reliability for the assessment of the distance travelled by the S-G cells at 6 and 12 hours was 0.91 (95% CI 0.658-0.976) between examiners AN and AK.
Results
Effects of vitamin D and vitamin D analog on cell viability
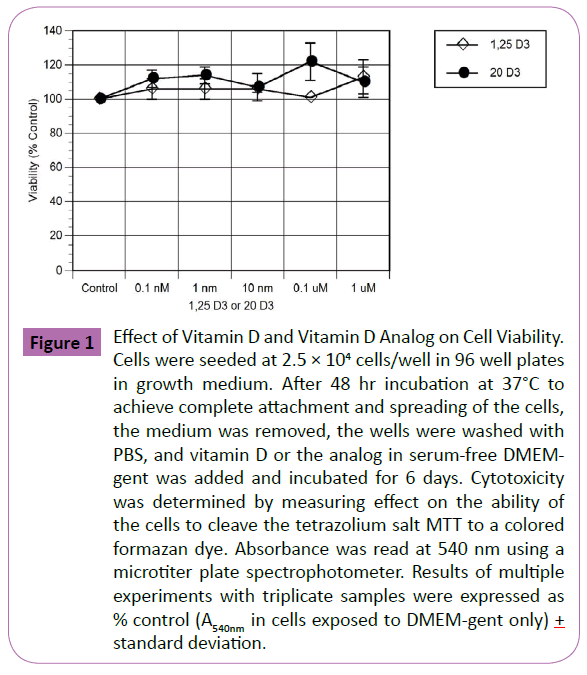
The effects of vitamin D and the vitamin D analog on cell viability were measured before determining their effects on other cellular functions. Exposure of S-G epithelial cells to both vitamin D and the analog at concentrations from 0.1 nM to 1 μM for 6 days had no significant effect on cell viability (Figure 1). While there was a trend of increasing activity with increased concentrations, these changes did not reach statistical significance. IC50 values for vitamin D and the analog were >1 μM. Previously, it was found that ethanol at ≤ 2.5% had no significant effect on S-G viability S. (Cho, D. Tipton, M. Dabbous, unpublished studies). We also tested DMEM containing 1% ethanol in the present study for effects on cell viability, and again, it did not have significant effects (data not shown). Therefore, in subsequent experiments, vitamin D and analog stock solutions made in absolute ethanol at 100x strength and diluted 1:100 in medium, so that the cells were exposed to 1% ethanol. Control media also contained 1% ethanol.
Figure 1: Effect of Vitamin D and Vitamin D Analog on Cell Viability. Cells were seeded at 2.5 × 104 cells/well in 96 well plates in growth medium. After 48 hr incubation at 37°C to achieve complete attachment and spreading of the cells, the medium was removed, the wells were washed with PBS, and vitamin D or the analog in serum-free DMEMgent was added and incubated for 6 days. Cytotoxicity was determined by measuring effect on the ability of the cells to cleave the tetrazolium salt MTT to a colored formazan dye. Absorbance was read at 540 nm using a microtiter plate spectrophotometer. Results of multiple experiments with triplicate samples were expressed as % control (A540nm in cells exposed to DMEM-gent only) + standard deviation.
Effects of vitamin D and vitamin D analog on LL- 37 production
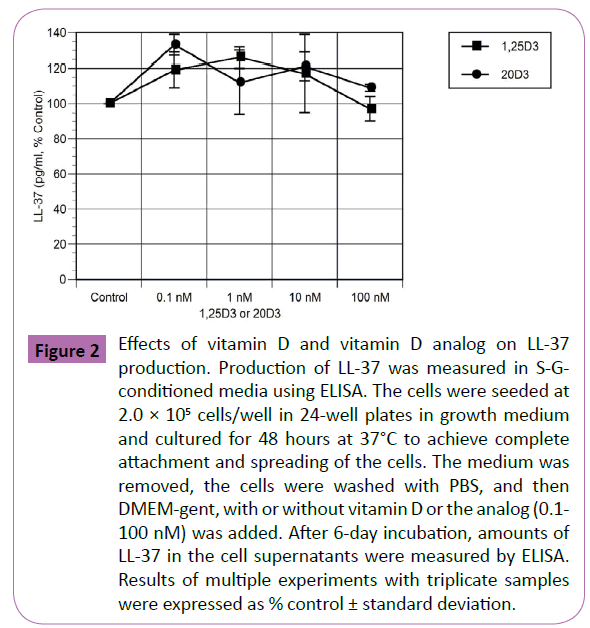
Control cultures of S-G cells produced 839 ± 20 pg/ml LL-37 after 6 days in culture. The effects of vitamin D and the analog, at concentrations of 0.1 nM to 100 nM, are shown in Figure 2, which shows the results of multiple experiments, expressed as percent control. While most concentrations of vitamin D and the analog increased LL-37 production in the range of 20-30%, these increases did not reach statistical significance. Similar results were seen at exposure times of 1 and 3 days (data not shown).
Figure 2: Effects of vitamin D and vitamin D analog on LL-37 production. Production of LL-37 was measured in S-Gconditioned media using ELISA. The cells were seeded at 2.0 × 105 cells/well in 24-well plates in growth medium and cultured for 48 hours at 37°C to achieve complete attachment and spreading of the cells. The medium was removed, the cells were washed with PBS, and then DMEM-gent, with or without vitamin D or the analog (0.1- 100 nM) was added. After 6-day incubation, amounts of LL-37 in the cell supernatants were measured by ELISA. Results of multiple experiments with triplicate samples were expressed as % control ± standard deviation.
Effects of vitamin D, vitamin D analog and neutralizing anti-LL-37 on proliferation
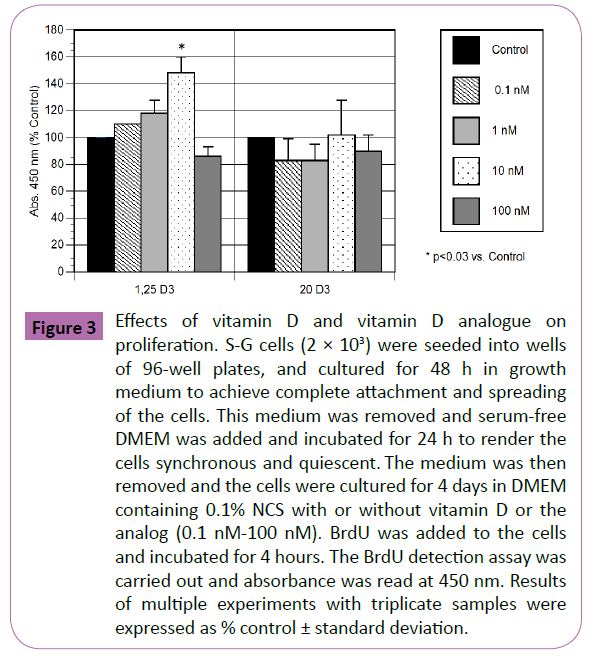
Vitamin D and the analog (0.1 nM -100 nM) were tested for their effects on S-G proliferation by measuring incorporation of BrdU into DNA (Figure 3). Concentrations of vitamin D from 0.1 nM to 10 nM increased proliferation, with a maximum at 10 nM (~50% increase; p<0.03), but the highest concentration caused ~15% decrease in proliferation, which was not significantly different from control. In contrast, the vitamin D analog caused no change or decreases in proliferation (~10-20%), which were not significant.
Figure 3: Effects of vitamin D and vitamin D analogue on proliferation. S-G cells (2 × 103) were seeded into wells of 96-well plates, and cultured for 48 h in growth medium to achieve complete attachment and spreading of the cells. This medium was removed and serum-free DMEM was added and incubated for 24 h to render the cells synchronous and quiescent. The medium was then removed and the cells were cultured for 4 days in DMEM containing 0.1% NCS with or without vitamin D or the analog (0.1 nM-100 nM). BrdU was added to the cells and incubated for 4 hours. The BrdU detection assay was carried out and absorbance was read at 450 nm. Results of multiple experiments with triplicate samples were expressed as % control ± standard deviation.
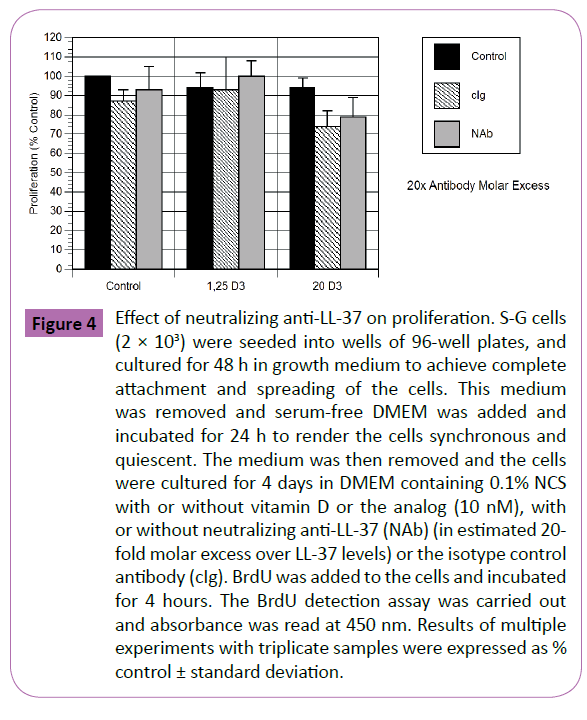
The effects of a neutralizing monoclonal antibody specific for human LL-37 (and an isotype control antibody) are shown in Figure 4. In these experiments, the cells were exposed to 10 nM vitamin D or analog, with or without the antibodies. In the presence of 20-fold molar excess anti-LL-37, there was no significant effect of the neutralizing antibody or the isotype control on proliferation (Figure 4). This was also true when the antibody was tested at 6-fold and 10-fold molar excess (data not shown).
Figure 4: Effect of neutralizing anti-LL-37 on proliferation. S-G cells (2 × 103) were seeded into wells of 96-well plates, and cultured for 48 h in growth medium to achieve complete attachment and spreading of the cells. This medium was removed and serum-free DMEM was added and incubated for 24 h to render the cells synchronous and quiescent. The medium was then removed and the cells were cultured for 4 days in DMEM containing 0.1% NCS with or without vitamin D or the analog (10 nM), with or without neutralizing anti-LL-37 (NAb) (in estimated 20- fold molar excess over LL-37 levels) or the isotype control antibody (cIg). BrdU was added to the cells and incubated for 4 hours. The BrdU detection assay was carried out and absorbance was read at 450 nm. Results of multiple experiments with triplicate samples were expressed as % control ± standard deviation.
In vitro wound assay
The results of the wound assay for gingival epithelial cell migration with vitamin D or the analog with and without the neutralizing antibody and the isotype control antibody, are shown in Figure 5 (normalized data shown from three independent experiments). Both time and treatment had a statistically significant effect on the distance travelled by S-G epithelial cells (p<0.001 and p<0.05 respectively). No difference was detected between all the vitamin D or the analog treatment modalities with or without the neutralizing antibody. The distance travelled with all the treatments of vitamin D and the analog were statistically significantly increased (p<0.05) at 12 hours compared to the ethanol control (approximately 3.36 fold). Although all treatments resulted in increased distance travelled by the S-G cells at 6 hours compared to the ethanol control (by approximately 3.03 fold), only the vitamin D analog group with the neutralizing antibody reached statistical significance at 6 hours (p<0.05).
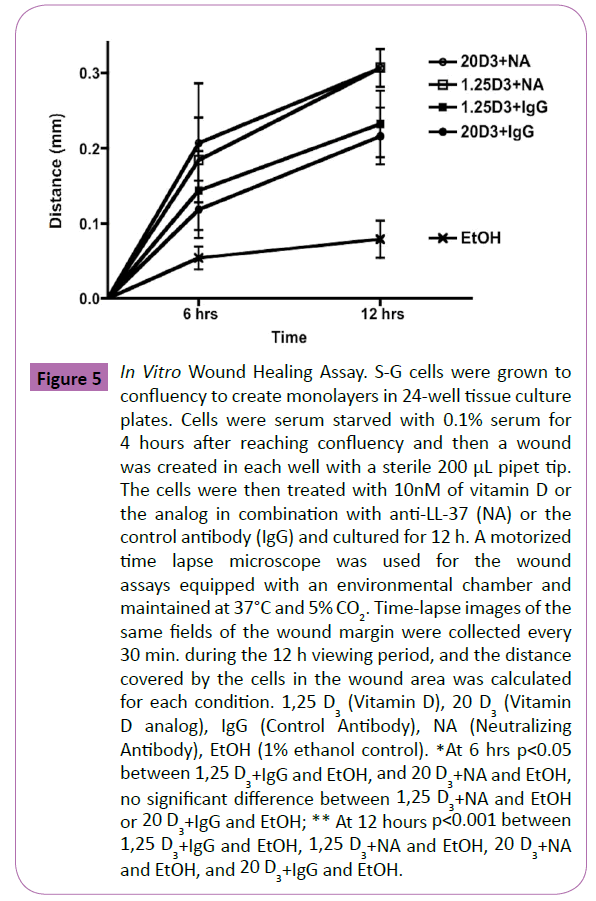
Figure 5: In Vitro Wound Healing Assay. S-G cells were grown to confluency to create monolayers in 24-well tissue culture plates. Cells were serum starved with 0.1% serum for 4 hours after reaching confluency and then a wound was created in each well with a sterile 200 μL pipet tip. The cells were then treated with 10nM of vitamin D or the analog in combination with anti-LL-37 (NA) or the control antibody (IgG) and cultured for 12 h. A motorized time lapse microscope was used for the wound assays equipped with an environmental chamber and maintained at 37°C and 5% CO2. Time-lapse images of the same fields of the wound margin were collected every 30 min. during the 12 h viewing period, and the distance covered by the cells in the wound area was calculated for each condition. 1,25 D3 (Vitamin D), 20 D3 (Vitamin D analog), IgG (Control Antibody), NA (Neutralizing Antibody), EtOH (1% ethanol control). *At 6 hrs p< 0.05 between 1,25 D3+IgG and EtOH, and 20 D3+NA and EtOH, no significant difference between 1,25 D3+NA and EtOH or 20 D3+IgG and EtOH; ** At 12 hours p< 0.001 between 1,25 D3+IgG and EtOH, 1,25 D3+NA and EtOH, 20 D3+NA and EtOH, and 20 D3+IgG and EtOH.
Discussion
Vitamin D and the vitamin D analog both stimulated LL-37 production by the S-G epithelial cells used in this study. Studies have shown that vitamin D exerts its effects on many tissues throughout the body because nearly every tissue displays the vitamin D receptors [36]. LL-37 is known to have a pleiotropic effect and has variety of physiological functions, including antimicrobial activity [5,6,14], strengthening the innate barrier [5], inhibiting osteoclastogenesis [7], suppressing pro-inflammatory cytokines, and enhancing anti-inflammatory markers [5]. Studies have shown that individuals with aggressive periodontitis had a local deficiency of LL-37 [37]. In the present study both vitamin D and the analog appeared to increase the stimulation of LL-37, which may offer benefits to patients with aggressive periodontitis.
Conflicting reports are emerging on whether vitamin D is proproliferative or anti-proliferative. Vitamin D appears to be antiproliferative in tumor cell lines [18,20,38,39] but stimulates proliferation in other types of cells [36,40,41]. When vitamin D and noncalcemic vitamin D analogs are used in the treatment of hyperproliferative skin disorders such as psoriasis, they have anti-proliferative effects [42]. Other studies have shown that vitamin D results in epidermal hyperplasia of human and mouse skin [41,43]. These conflicting reports may be related to the type of cell studied, concentration effects, factors present in the local environment, the medium being used, or the amounts of growth factors in the medium [41]. The analog used in this study previously was shown to have an anti-proliferative effect on tumor cells [18] and it also was slightly anti-proliferative to S-G epithelial cells.
The present study showed that vitamin D has a biphasic effect on gingival epithelial cell growth in vitro. Concentrations less than or equal to 10 nM stimulated cell proliferation under the conditions used in this study. However, 100 nM vitamin D suppressed cell growth. Similar results have been demonstrated in other studies [44]. The vitamin D analog used in this study did not follow this pattern of effect on proliferation, and actually appeared to be anti-proliferative in most concentrations used with this cell line. Although the analog did not stimulate proliferation, it demonstrated the same increase of wound closure as did vitamin D, indicating the mechanism of wound closure may involve an increase in the migratory capacity of the cells, rather than increased growth rates.
Vitamin D has been shown to be beneficial in wound healing [45]. Many proposed mechanisms of the benefits of vitamin D in wound healing involve the activation of the vitamin D receptor and increased production of human cathelicidin LL-37. The increase in LL-37 may prove to be beneficial due to its ability to exert an antimicrobial effect and to reduce production of proinflammatory cytokines. In this study, we could not confirm that LL-37 is necessary to increase the rate of wound closure in an in vitro setting. Mechanisms other than LL-37 stimulation which cause an increase in the proliferation and migration of cells treated with vitamin D have been suggested. For example, Molinari [36] showed that an increase in proliferation and migration occurs in vitamin D supplementation involving a mechanism of vitamin D-induced eNOS dependent nitric oxide production. More investigation is needed to determine whether the increase in epithelial wound closure caused by vitamin D or the analog involves the nitric oxide pathway in S-G cells.
Our work showed that the vitamin D and the vitamin D analog increased the rate of wound healing in an in vitro model, with approximately 3.36 fold increase in wound closure at 12 hours compared to the control. This was not dependent on LL-37, and in the case of the analog, the increased rate of wound healing was not related to proliferation. Wong et al. have shown that vitamin D promotes vascular regeneration through mechanisms involving a chemotactic effect on angiogenic myeloid cells [46]. They suggested that a positive effect of vitamin D on re-endothelialization may be a consequence of promoting endothelial migration. Kogawa showed that vitamin D augmented the cell migration of giant cell tumor of bone [47], and Bakdash demonstrated that vitamin D increased migration of CD14+ dermal dendritic cells [48]. All this emerging evidence that vitamin D affects cell migration may suggest that the gingival epithelial cells used in this study showed an increase rate of wound closure when supplemented with vitamin D or the vitamin D analog due to an increase in the migration of the cells, regardless of the levels of LL-37.
The reason an analog was used in this study is that there has been controversy concerning the adequate amount of vitamin D required by individuals. Recent studies suggest that the daily required amount of vitamin D should be elevated to maximize its beneficial health effects. The Office of Dietary Supplements recommends that serum 25-hydroxy vitamin D levels in the serum should be greater than 50 nmol/L and the Institute of Medicine reported that levels above 125 nmol/L may potentially have adverse effects. The concentration of vitamin D used in this study was 10 nmol/L, which is 5 fold less than the optimum physiologic level. The major concern of elevated levels of vitamin D is related to hypercalcemia [49-51]. Animal studies involving vitamin D have shown that serum concentrations of 25-hydroxy vitamin D3 of 2.5 micromol/L can be accompanied by hypercalcemia [18,50]. Studies have demonstrated the efficacy of analogs of vitamin D (20-hydroxyvitamin D2 and 20-hydroxyvitamin D3), which has the ability to activate the vitamin D receptor yet not induce any significant elevation of serum calcium levels in animal models [18,52]. While 2 μg/kg of 1,25 dihydroxyvitamin D3 and 25 hydroxyvitamin D2 induced significantly elevated amounts of calcium in the serum in an animal study, doses of up to 30 μg/kg of 20 hydroxyvitamin D3 did not significantly alter serum calcium levels [18]. The use of such analogs may prove advantageous in a clinical setting in order to maximize the benefits of vitamin D without subjecting the patient to hypercalcemia.
Although the vitamin D did cause a significant increase in the proliferation of the cell line at a concentration of 10 nM, this was not the case for the vitamin D analog which at some concentrations was anti-proliferative. Vitamin D and the vitamin D analog in this study at concentrations of 10 nM both increased LL-37 production, as well as the rate of in vitro wound healing, characteristics which may prove to be beneficial in conjunction with traditional periodontal treatment modalities when treating periodontal disease.
Acknowledgements
This work was done in partial fulfillment of the requirements for the degree of Master of Dental Science (AN). The authors thank the University of Tennessee College of Dentistry Alumni Endowment Fund and The Tennessee Dental Association Foundation for their support for this research project. Partial support of the NIH grant 1R21AR0666505-01A1 (AS) is also acknowledged. The authors thank Dr. Franklin Garcia-Godoy, senior executive associate dean or research, for the use of the time lapse microscope (Zeiss AXIO Observer Z1, Zeiss 102404853, Jena, Germany) which was used for the wound assays.
References
- Stein SH, Livada R, Tipton DA (2014) Re-evaluating the role of vitamin D in the periodontium. J Periodont Res 49: 545-553.
- Heilborn JD, Weber G, Gronberg A, Dieterich C, Stahle M (2010) Topical treatment with the vitamin D analogue calcipotriol enhances the upregulation of the antimicrobial protein hcAP18/LL-37during wounding in human skin in vivo. Exp Dermatol 19: 332-338.
- White JH (2010) Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: Past, present, and future. J Steroid Biochem Molec Biol 121: 234-238.
- Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, et al. (2007) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117: 803-811.
- Vander AM, Bergman P, Agerberth B, Lindbom L (2012) Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol 9: 735-742.
- Schauber J, Gallo R (2008) Antimicrobial peptides and the skin immune defense system. J Allerg Clin Immunol 122: 261-266.
- Supanchart C, Thawanaphong S, Makeudom A, Bolscher JG, Nazmi K, et al. (2012) The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dental Res 91: 1071-1077.
- Carretero M, Escamez M, Garcia M, Duarte B, Holguin A, et al. (2008) In vitro and In vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol 128: 223-236.
- Ramos R, Silva JP, Rodrigues AC, Costa R, Guardao L, et al. (2011) Wound healing activity of the human antimicrobial peptide LL37. Peptides 32: 1469-1476.
- Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa, H, Ouhara K, et al. (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 175: 4662-4668.
- Sato-Deguchi E, Imafuku S, Chou B, Ishii K, Hiromatsu K, et al. (2012). Topical vitamin D (3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol 167: 77-84.
- Inagaki K, Krall EA, Fleet JC, Garcia RI (2003) Vitamin D receptor alleles, periodontal disease progression, and tooth loss in the VA dental longitudinal study. J Periodontol 4: 161-167.
- Bashutski JK, Eber RM, Kinney JS, Benavides E, Maitra S, et al. (2011) The impact of vitamin D status on periodontal surgery outcomes. J Dent Res 90: 1007-1012.
- McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, et al. (2011) Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun 79: 2250-2256.
- Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, et al.(2009) Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol 80: 1433-1439.
- Alshouibi EN, Kaye EK, Cabral HJ, Leone CW, Garcia RI (2013) Vitamin D and periodontal health in older men.J Dent Res 92: 689-693.
- Boggess K, Espinola J, Moss K, Beck J, Offenbacher S, et al. (2011). Vitamin D status and periodontal disease among pregnant women. J Periodontol 82: 195-200.
- Wang J, Slominski A, Tuckey R, Janjetovic Z, Kulkarni A, et al. (2012) 20-Hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res 32: 739-746.
- Chen J, Wang J, Kim T, Tieu EW, Tang EKY, et al. (2014) Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and anti-proliferative activity. Anticancer Res 34: 2153-2163.
- Janjetovic Z, Tuckey R, Nguyen M, Thorpe E, Slominski A (2010) 20,23-Dihydroxyvitamin D3, novel p450scc product, stimulates differentiation and inhibits proliferation and NF-kB activity in human keratinocytes. J Cell Physiol 223: 36-48.
- Carroll M, Schade D (2003) A practical approach to hypercalcemia. AmFam Physician 67:1959-1966.
- Slominski AT, Kim TK, Shehabi HZ, et al. (2012) In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J 26: 3901-3915.
- Slominski A, Semak I, Zjawiony J, et al. (2005) The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 272: 4080-4090.
- Smulow J, Glickman I (1966)An epithelial-like cell line grown in continuous culture from normal adult human gingiva. ProcSocExpBiol Med 121: 1294-1296.
- Kasten FH, Pimeda LFR, Schneider PE, Rawls HR, Foster TA (1989)Biocompatibility testing of an experimental fluoride releasing resin using human gingival epithelial cells in vitro. In Vitro Cell Dev Biol 25: 57-62.
- Kasten FH, Seileu K, Nefert RM (1990) Quantitative evaluation of human gingival epithelial cell attachment to implant surfaces in vitro. Int J Restor Dent 10: 69-79.
- Rawls HR, Starr J, Kasten FH, Murray M, Smid J, et al. (1990) Radiopaque acrylic resins containing miscible heavy metal compounds.Dent Mater 6: 250-255.
- Tipton DA, Hamman NR, Dabbous MKh (2006) Effect of myrrh oil on IL-1β stimulation of NF-kB activation and PGE2 production in human gingival fibroblasts and epithelial cells.Toxicol In Vitro 20: 248-255.
- Tipton DA, Lewis JL (2008) Effects of a hindered amine light stabilizer and a UV light absorbed used in maxillofacial elastomers on human gingival epithelial cells and fibroblasts.J Prosthet Dent 100: 220-231.
- Tipton DA, Lyle B, Babich H, DabbousMKh (2003) In vitro cytotoxic and anti-inflammatory effects of myrrh oil on human gingival fibroblasts and epithelial cells. ToxicolIn Vitro 17: 301-310.
- Babich H, Tipton DA (1999) In vitro cytotoxicity of bisphenol A to human gingival epithelial S-G cells.In VitroMolToxicol 12: 233-244.
- Babich H, Tipton, DA (2002) In vitro response of human gingival epithelioid S-G cells to minocycline. ToxicolIn Vitro 16: 11-21.
- Tobey R, Valdez J, Crissman H (1988) Synchronization of human diploid fibroblasts at multiple stages of the cell cycle. Exp Cell Res 179: 400-416.
- Denker SP, Barber DL (2002) Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol 159: 1087-1096.
- Frantz C, Karydis A, Nalbant P, Hahn KM, Barber DL (2007) Positive feedback between Cdc42 activity and H+ efflux by the Na-H exchanger NHE1 for polarity of migrating cells. J Cell Biol 179: 403-410.
- Molinari C, Rizzi M, Squarzanti DF, Pittarella P, Vacca G, et al. (2013) 1α,25 Dihydroxycholecalciferol (Vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cell PhysiolBiochem31: 815-822.
- Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J (2008) Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37in the innate immune response against periodontogenic bacteria. Oral MicrobiolImmunol 23: 328-335.
- Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL (2002) Antiproliferative effects of 1alpha, 25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology 143: 2508-2514.
- Chung I, Han G, Seshadri M, Gillard BM, Yu WD, et al. (2009) Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res 69: 967-975.
- Ramesh KV, Mahindrakar MB, Bhat EP (1993) A new role for vitamin D: cholecalciferol promotes dermal wound strength and re-epithelization. Indian J ExpBiol 31: 778-779.
- Gniadecki R, Serup J (1995) Stimulation of epidermal proliferation in mice with 1,25-dihydroxyvitamin D3 and receptor-active 20- EPI analogues of 1,25-dihydroxyvitamin D3. BiochemPharmacol 49: 621-624.
- Walters MR (1992) Newly identified actions of the vitamin D endocrine system. Endocr Rev 13: 719-764.
- Levy J, Gassmuller J, Schroder G, Audring H, Sonnichsen N (1994) Comparison of the effects of calcipotriol, prednicarbate and clobetasol 17-propionate on normal skin assessed by ultrasound measurement of skin thickness. Skin Pharmacol 7: 231-236.
- Gurlek A, Pittelkow MR, Kumar R (2002) Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25- dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev 23: 763-786.
- Elizondo RA, Yin Z, Lu X, Watsky MA (2014) Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Invest Opthalmol Vis Sci 55: 5245-5251.
- Wong M, Leisegang M, Kruse C, Vogel J, Schurmann C, et al. (2014) Vitamin D promotes vascular regeneration. Circulation 130: 976-986.
- Kogawa M, Findlay DM, Anderson PH, Atkins GJ (2013) Modulation of osteoclastic migration by metabolism of 25OH-vitamin D3. J Steroid BiochemMolBiol 136: 59-61.
- Bakdash G, Schneider LP, Capel TM, Kapsenberg ML, Teunissen MB, et al. (2013) Intradermal application of vitamin D3 increases migration of CD14+ dermal dendritic cells and promotes the development of Foxp3+ regulatory T cells. Hum VaccinImmunother 9: 250-258.
- Pandita K, Razdan S, Kudyar RP, Beigh A, Kuchay S, et al. (2012) Excess good can be dangerous. A case series of iatrogenic symptomatic hypercalcemia due to hypervitaminosis D. Clin Cases Min Bone Metab 9:118-120.
- Jones G (2008) Pharmacokinetics of vitamin D toxicity. Am J ClinNutr 88: 582S-586S.
- Kilpatrick RD, Danese MD, Belozeroff V, Smirnakis K, Goodman WG, et al. (2011) The association of vitamin D use with hypercalcemia and hyperphosphatemia in hemodialysis patients: a case-crossover study. Pharmacopeidemiol Drug Safety 20: 914-921.
- Slominski A, Kim T, Janjetovic Z, Tuckey RC, Bieniek R, et al. (2011) 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol 300: C526-C541.