- (2009) Volume 10, Issue 6
Banavara Narasimhamurthy Girish2, Gopalakrishna Rajesh1, Kannan Vaidyanathan3, Vallath Balakrishnan1
Departments of 1Gastroenterology, 2Physiology, and 3Biochemistry; Amrita Institute of Medical Sciences. Cochin, Kerala, India
Received: 12 March 2009 Accepted: 13 August 2009
Context A major role of the pancreas in zinc homeostasis has been suggested. Objective To assess erythrocyte zinc status in chronic pancreatitis and to correlate it with pancreatic exocrine and endocrine insufficiency. Patients One hundred and one patients with chronic pancreatitis (34 alcoholic chronic pancreatitis, 67 tropical chronic pancreatitis) were prospectively studied. Main outcome measure Disease characteristics and imaging features were recorded. Erythrocyte zinc was estimated by flame atomic absorption spectrophotometry. Exocrine insufficiency was assessed using polyclonal antibody ELISA for pancreatic stool elastase1. Endocrine insufficiency was assessed by serum glucose levels and insulin requirement. Results Erythrocyte zinc was significantly lower in chronic pancreatitis patients than in the controls (26.5±9.5 μg/g Hb vs. 38.0±6.6 μg/g Hb; P<0.001), and in tropical chronic pancreatitis than in alcoholic chronic pancreatitis (25.0±10.4 μg/g Hb vs. 29.6±6.5 μg/g Hb, P=0.001). In chronic pancreatitis patients who had exocrine insufficiency, erythrocyte zinc positively correlated with stool elastase1 (r=0.587, P<0.001). Erythrocyte zinc levels were significantly lower in diabetic patients as compared to non-diabetics (P=0.036). Conclusions This study demonstrates zinc deficiency in chronic pancreatitis patients, and that zinc deficiency correlates with exocrine and endocrine insufficiency. Further studies may clarify the possible benefits of zinc supplementation in chronic pancreatitis.
Diabetes Mellitus; Pancreatic Elastase; Pancreatitis, Alcoholic; Zinc
Unlike the West, in India the predominant form of chronic pancreatitis remains idiopathic chronic pancreatitis, which also includes tropical pancreatitis despite the well-documented rise in alcoholic chronic pancreatitis [1]. There still remains a lack of clear understanding on the etiopathogenesis of tropical chronic pancreatitis although various theories namely malnutrition, dietary toxins and micronutrient deficiency etc., have been proposed, but none have been proven. Furthermore, tropical chronic pancreatitis was classically seen to have an earlier onset, a rapid onset of diabetes and, probably, a more deleterious course although current studies do indicate a trend to a more delayed onset [2].
A high zinc turnover in the pancreas has been reported [3, 4]. Endogenous zinc was seen to be excreted by pancreatic exocrine secretions [5]. In addition, pancreatic acinar cells appeared to have very high 65Zn turnover rates as compared to islet tissue [6]. The relationship of diabetes mellitus and zinc is complex [7]. Dominguez-Munoz et al. have tried to examine the role of quantification of pancreatic zinc output as a measure of pancreatic function and have found a sensitivity and specificity of 97% and 91%, respectively, in patients with chronic pancreatitis in comparison to the secretin-cerulein test [8]. However, this method has the same drawbacks as the standard direct pancreatic exocrine function tests of being cumbersome to perform. On the other hand, Pungapong et al. did not find any significant change in zinc output in pancreatic fluid in chronic pancreatitis patients. The reason for this difference was postulated to be due to differences in methodology and the severity of the disease between the two patient groups [9]. There have been no reports in the literature regarding zinc status in tropical chronic pancreatitis while only a few reports exist on the relationship of zinc status with pancreatic exocrine insufficiency and diabetes in chronic pancreatitis [10, 11].
This study was undertaken to assess zinc status in alcoholic and tropical chronic pancreatitis and also to examine its relationship to pancreatic exocrine and endocrine insufficiency.
Patients
Chronic pancreatitis patients were recruited from the Pancreas Clinic, Amrita Institute of Medical Sciences. Chronic pancreatitis was defined by features consistent with irreversible pancreatic inflammation, i.e., clinical, structural or functional abnormality of the pancreas [12]. The presence of pancreatic calculi or ductal irregularity/parenchymal atrophy was determined at imaging using ultrasonography, CT scan, MRI, magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP) or endoscopic ultrasound (EUS). Patients having chronic pancreatitis with an alcohol consumption equal to, or greater than, 80 g/day for at least 5 years were considered to have alcoholic chronic pancreatitis while tropical chronic pancreatitis was defined using previously reported criteria [1].
One hundred and one patients with chronic pancreatitis (34 alcoholic chronic pancreatitis, 67 tropical chronic pancreatitis) were included and prospectively studied (Table 1).
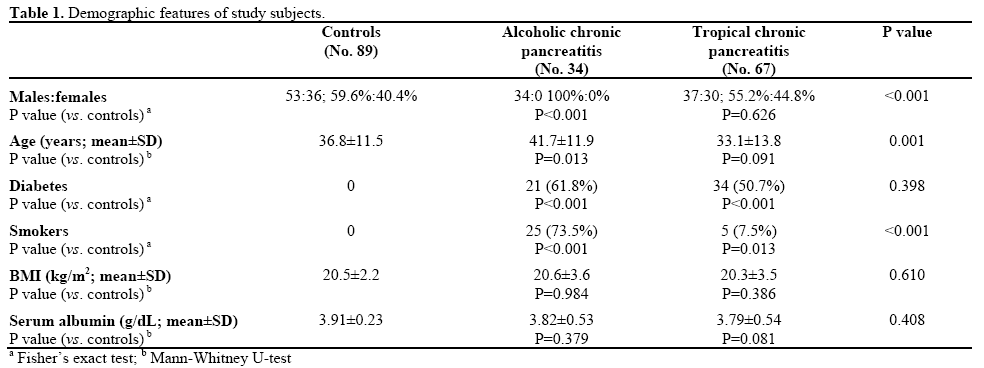
Eighty-nine controls (53 males, 36 females) were recruited from healthy hospital visitors. None of the patients or controls had a frank diarrhea.
Dietary details of each subject were collected and recorded. Subjects using vitamin and mineral supplements, or consuming fortified food, were excluded from the study.
Disease characteristics, such as pain, steatorrhea, diabetes mellitus and insulin requirements as well as risk factors, such as alcohol and smoking, and imaging (US/CT) features, such as calculi, parenchymal atrophy and ductal dilation, were recorded. BMI was also calculated (weight in kg / squared height in m2).
Serum albumin was measured using bromocresol green [13]. Erythrocyte zinc was estimated as it provides an assessment of zinc status over a longer period of time as compared to that of the rapidly turning over plasma pool [14]. Fasting blood samples were collected in heparinized vacutainers. RBCs were washed 3 times by resuspending the cells in cold normal saline and centrifuging (1,500 g at 4°C). The buffy coat was separated. The washed cells were lysed with two volumes of milli-Q water and frozen at -20°C. Hemoglobin concentration was measured using the cyanmethhemoglobin method. Erythrocyte lysate was diluted 10 fold with milli-Q water; zinc concentration was determined by flame atomic absorption spectrophotometry (3110, Perkin Elmer, Waltham, MA, USA) [15] and values were expressed as micrograms of zinc per g of hemoglobin
Stool samples of chronic pancreatitis patients were collected and stored at -4°C for less than one week prior to use. Pancreatic stool elastase1 was measured by using a polyclonal antibody-based ELISA kit (Bioserv, Rostock, Germany). Stool elastase1 in moderate pancreatic exocrine insufficiency was 100 to 200 μg/g and, for severe exocrine insufficiency, it was less than, or equal to, 100 μg/g.
Plasma fasting and postprandial glucose levels and insulin requirements were recorded to estimate endocrine insufficiency. Diabetes mellitus was diagnosed if the fasting serum glucose value was equal to, or greater than, 126 mg/dL confirmed on two occasions and/or a serum glucose value equal to, or greater than, 200 mg/dL after a two-hour glucose load confirmed on two occasions, and/or requirements for insulin or oral hypoglycemic drugs.
Written informed consent was obtained from all study subjects. The study protocol conforms to the ethical guidelines of the "World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, as revised in Tokyo 2004. The study was approved by the institutional ethical review committee.
Statistical analysis was conducted by using SPSS (version 11). Data are reported as mean±SD and frequencies. Odds ratios (ORs) were evaluated together with their 95% confidence intervals (CIs). The Mann- Whitney U-test test was performed to compare means. Pancreatic stool elastase1 levels were reported as median and interquartile ranges. The Fisher’s exact test was used to compare the percentages of the dichotomous variables and a linear correlation between the two groups was evaluated by calculating the Spearman rank correlation coefficient. The ROC curve was calculated to evaluate the accuracy of RBC zinc concentration in predicting low pancreatic stool elastase1; the area under the ROC curve was computed together with the standard error (AUC±SE) and the 95% CI. The optimal cut-off value which best predicted low elastase1 was calculated using a maximum likelihood ratio method [16]. Two-tailed P values less than 0.05 were considered statistically significant.
The mean age of the alcoholic chronic pancreatitis patients (34 male, 0 females) was 41.7±11.9 years and that of the tropical chronic pancreatitis patients (37 males, 30 females) was 33.1±13.8 years. The mean age of the controls (53 males and 36 females) was 36.8±11.5 years.
Zinc in Chronic Pancreatitis (Alcoholic Chronic Pancreatitis vs. Tropical Chronic Pancreatitis)
Erythrocyte zinc levels were significantly reduced in chronic pancreatitis patients as compared to the controls (P<0.001; Figure 1). Zinc values were significantly lower in patients with tropical chronic pancreatitis as compared to alcoholic chronic pancreatitis patients (P=0.001).
Zinc and Nutritional Status
The majority of our chronic pancreatitis patients was consuming a non-vegetarian diet and dietary items rich in zinc content and was unlikely to have any significant zinc deficiency in their diet. None of the patients had any overt clinical features to suggest zinc deficiency. The nutritional status and BMI of patients with alcoholic and tropical chronic pancreatitis were comparable to the controls (Table 1). There was no significant correlation between BMI and erythrocyte zinc concentration in chronic pancreatitis patients (r=- 0.072, P=0.542).
Serum albumin was lower in alcoholic chronic pancreatitis and tropical chronic pancreatitis patients as compared to the controls but the difference was not statistically significant (Table 1). Serum albumin did not correlate with BMI (r=0.172, P=0.431) or RBC zinc (r=0.013, P=0.813). Low serum albumin (less than 3.5 g/dL) was associated with low pancreatic stool elastase1 (26/67, 38.8% vs. 4/34, 11.8% in patients with and without pancreatic exocrine insufficiency, respectively; OR=4.76, 95% CI: 1.50-15.07, P=0.005).
Zinc and Pancreatic Exocrine Insufficiency
Pancreatic exocrine insufficiency (fecal elastase1 equal to, or less than, 200 μg/g stool) was observed in 67 (66.3%) chronic pancreatitis patients. Moderate (101- 200 μg/g stool) and severe (0-100 μg/g stool) insufficiency was seen in 13 (12.9%) and 54 (53.5%) patients respectively. However median elastase1 levels were comparable in alcoholic and tropical chronic pancreatitis (133 μg/g stool vs. 99 μg/g stool, respectively; P=0.516) (Figure 2).
Erythrocyte zinc positively correlated with pancreatic stool elastase1 (r=0.587, P<0.001) (Figure 3). Erythrocyte zinc levels were significantly lower in patients with low elastase1 levels as compared to those with normal stool elastase1 (23.8± 8.6 vs. 32.1±9.0, P<0.001).
Erythrocyte zinc levels in chronic pancreatitis patients with normal elastase1 were significantly lower than in the controls (32.1±9.0 vs. 38.0±6.6 μg/g Hb, P<0.001). To evaluate the performance of RBC zinc in diagnosis of pancreatic insufficiency, we calculated a ROC curve (Figure 4) by using RBC zinc concentration in predicting low pancreatic stool elastase1. We obtained an area under the ROC curve (AUC±SE) of 0.739±0.065 (95% CI: 0.611-0.866). At the best cut-off value of RBC zinc (28.5 μg/g Hb), the sensitivity and specificity were 82.1% (55/67) and 61.8% (21/34), respectively.
Zinc and Diabetes
Erythrocyte zinc levels were significantly lower in diabetic patients as compared to non-diabetics (P=0.036) (Figure 1).
Forty-one (61.2%) of the 67 patients with low elastase1 were diabetic in comparison to 14 (41.2%) of the 34 patients with normal elastase1 levels (P=0.062). In addition, 35 (64.8%) of the 54 chronic pancreatitis patients with pancreatic stool elastase1 less than, or equal to, 100 μg/g stool were diabetic as compared to 20 (42.6%) of the 47 chronic pancreatitis patients with pancreatic stool elastase1 greater than 100 μg/g stool (P=0.029). No significant difference in the prevalence of low elastase1 was noted between diabetic chronic pancreatitis patients who required insulin for glycemic control (12/15, 80.0%) and those who did not (29/40, 72.5%) (P=0.734).
In this study, we observed a positive correlation of erythrocyte zinc and elastase1 levels in chronic pancreatitis patients, the latter being a measure of pancreatic exocrine function. A caveat is that determination of pancreatic exocrine insufficiency using fecal pancreatic elastease1 can underestimate its true prevalence, as the test is not very sensitive in mild exocrine insufficiency [17]. However, the test is easily the best available indirect test and is a reliable test for detecting clinically relevant pancreatic exocrine insufficiency [18].
Pancreatic juice contains zinc in high concentrations as a constituent of metalloenzymes, such as carboxypeptidase and carbonic anhydrase. It has been suggested that the pancreas plays a major role in zinc homeostasis [19]. Previously, Boosalis et al. [20] proposed that pancreatic exocrine insufficiency can alter zinc metabolism. Evans et al. [21] have reported that the pancreas secretes a low molecular weight zinc binding ligand which facilitates zinc absorption from the intestines of rats. Altered zinc absorption in chronic pancreatitis may be related to reduced concentrations of ligands in the pancreatic juice. Dutta et al. have shown increased zinc excretion in patients with pancreatic exocrine insufficiency [10]. Hence, a negative zinc balance exists in patients with pancreatic exocrine insufficiency [22].
On the other hand, zinc deficiency could influence pancreatic function. Ultrastructural studies of pancreatic acinar cells in rats fed a zinc-deficient diet showed the destruction of zymogen granules and lysosomes. This indicates that zinc has a role in maintaining the structural integrity of pancreatic acinar cells [23]. Rats with a marginal zinc deficiency show morphological and functional alterations in the pancreas similar to those induced by alcohol [24]. Zinc deficiency may play a role in ethanol induced secretory alterations, because a poor intake of zinc is often associated with chronic alcoholism [25]. Furthermore, the increased activity of prolyl hydroxylase, an enzyme which takes part in the synthesis of collagen, in experimentally zinc-depleted pancreatic tissue suggests that zinc deficiency is associated with pancreatic collagen deposition and may thus be implicated in pancreatic fibrosis [26]. It has recently been noted that Coxsackievirus B3-infected mice showed significant reduction in pancreatic zinc and metallothionein 1 content and it has been suggested that this may reflect the early stages of the development of pancreatitis [27]. Thus, pancreatic function and zinc status appear to be mutually dependant.
Zinc deficiency may be the effect of reduced absorption and can be a contributory factor in disease progression, via the reduction of free radical scavengers, increased oxidant stress and increased collagen deposition. Other possible effects could be an alteration in immune function.Our study is the first to estimate zinc levels in tropical chronic pancreatitis. The observation of significantly lower levels of erythrocyte zinc in tropical chronic pancreatitis as compared to alcoholic chronic pancreatitis has important implications, despite its limitations in terms of disparity in numbers between the two groups. The relationship of this observation to the concept of micronutrient deficiency in the etiopathogenesis of tropical chronic pancreatitis needs to be examined more closely. This is possible through more elaborate studies designed to measure the zinc balance, including ingestion and excretion of zinc using radiolabeled markers in an attempt to arrive at a cause-effect relationship. Furthermore, a deficiency of methionine and cysteine, which has been implicated in the etiopathogenesis of tropical chronic pancreatitis, may play a role in the alteration of zinc status in tropical chronic pancreatitis as these sulphur aminoacids are known to facilitate zinc absorption. We presume that zinc deficiency could be due to pancreatic exocrine insufficiency as evidenced by the direct correlation between erythrocyte zinc and pancreatic stool elastase1. However, diabetes has been shown to be an independent risk factor for hypozincemia [11, 28]. This observation is in agreement with our own observation of lower zinc levels in diabetic patients with chronic pancreatitis as compared to non-diabetic patients with chronic pancreatitis. This effect of diabetes on zinc levels may probably be more marked in tropical chronic pancreatitis as compared to alcoholic chronic pancreatitis, but can only be confirmed in a larger series of patients. Furthermore, zinc has important subcellular metabolic functions and is involved in the synthesis, storage and secretion of insulin as well as the conformational integrity of insulin in the hexameric form [7]. Zinc deficiency may potentiate oxidative stress and is a likely factor in the complications of diabetes. It has been shown that the endocrine pancreas is able to compensate for subclinical zinc deficiency in rats as it maintains an adequate zinc ion level in the secretory vesicles for insulin storage. The exocrine pancreas lacks this ability; it exhibits decreased levels of zinc ion staining as a consequence of 4 weeks of reduced zinc intake [29].
The relationship between zinc status vis a vis pancreatic exocrine and endocrine insufficiency appears to be complex. Our study clearly demonstrates a zinc deficiency in chronic pancreatitis and its close correlation with both exocrine and endocrine insufficiency. It would be interesting to see the differential effects on exocrine and endocrine function in patients with chronic pancreatitis, treated with zinc supplementation, in an appropriately designed study. The clinical benefits of zinc supplementation in chronic pancreatitis, especially tropical chronic pancreatitis, can be better elucidated by a case control study.
We acknowledge the financial support from the Kerala State Council for Science
Technology and Environment, India and the technical assistance of Dr. Chandramohan Kumar, Cochin University of Science and Technology (CUSAT), Kerala, India, for the metal analysis. We would alsolike to thank Dr Karimassery Ramaiyer Sundaram, Professor and Head of Department of Biostatistics, Amrita Institute of Medical Sciences, Cochin, for his help with the statistical analysis.
Conflict of interest The authors have no potential conflicts of interest